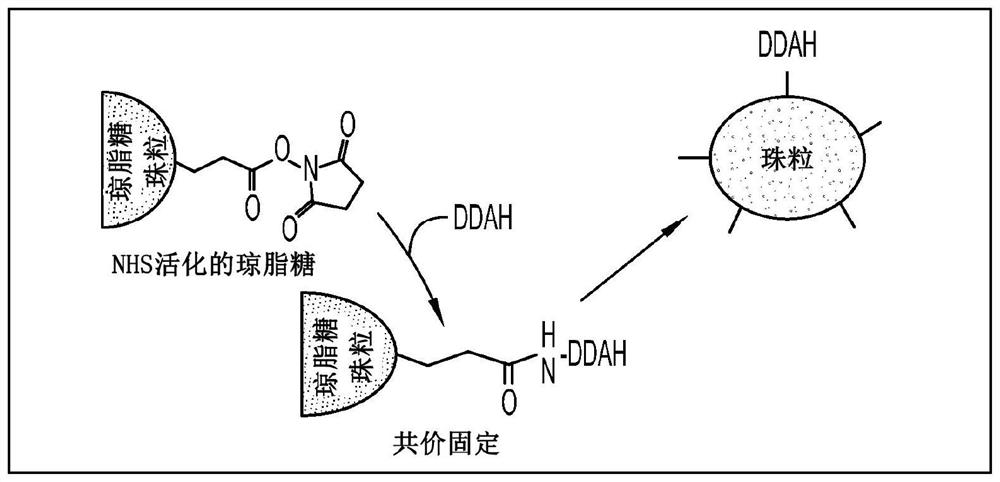
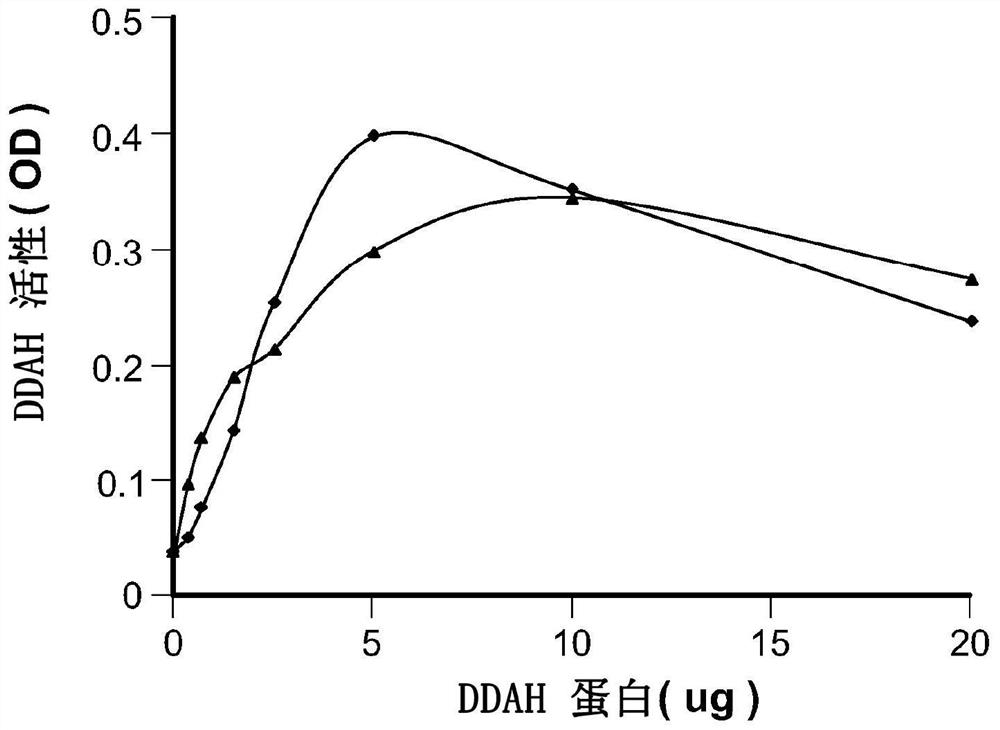
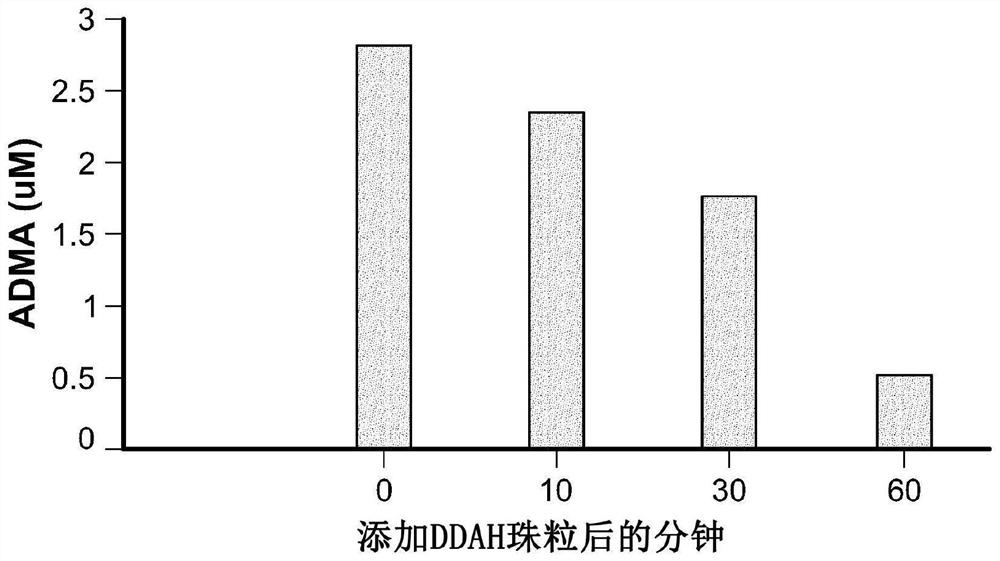
Device and method for modulating adma in blood
A technology for blood and blood treatment, which can be used in combination or as a component of a hemodialysis system or in the field of plasma, and can solve problems such as the reduction of ADMA clearance rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0167] The major route of elimination of ADMA from the body is through the enzymatic action of DDAH. In patients with a wide variety of diseases and conditions (eg renal disease, coronary artery disease, congestive heart failure, hypertension, pulmonary hypertension, and especially end-stage renal failure, surgical patients, trauma patients, intensive care unit patients) Elevated ADMA levels were found in . ADMA levels were also increased in patients with acute kidney injury and contrast-induced kidney injury. Additionally, increased ADMA levels have been reported to be an indicator of cardiovascular-related mortality risk.
[0168] High levels of ADMA and reduced DDAH are found in patients with preeclampsia, which can lead to high blood pressure, kidney damage, reduced fetal growth, and preterm birth. High levels of ADMA are associated with erythropoietin resistance.
[0169] Therefore, there is an urgent need to develop methods for reducing the concentration of ADMA in th...
Embodiment 1
[0205] Recombinant expression and purification of human DDAH
[0206] Methods for cloning DDAH, such as polypeptide and polynucleotide sequences of DDAH and cloning DDAH into host cells, are known to those of ordinary skill in the art. The cDNAs encoding human DDAH 1 and DDAH 2 are set forth in SEQ ID NO: 3 and SEQ ID NO: 4, and the amino acid sequences of human DDAH 1 and DDAH 2 polypeptides are set forth in SEQ ID NO: 1 and SEQ ID NO: 2. The non-human amino acid sequences of DDAH polypeptides are set forth in SEQ ID NOs: 6; 7; 9; 10; 11; Biosynthesis of DDAH polypeptides is accomplished by transforming E. coli with a plasmid comprising the nucleotide sequence of DDAH or a modified DDAH or DDAH analog.
[0207] Wild-type mature DDAH was amplified by PCR from a cDNA synthesis reaction using standard protocols and cloned into pET30 (NcoI-BamHI). The nucleic acid sequence encoding DDAH is subcloned into an expression vector under the constitutive or inducible control of a synt...
Embodiment 2
[0211] E. coli expresses DDAH polypeptides.
[0212] E. coli strain W3110 was used to produce wild-type or modified DDAH. A single Research Cell Bank (RCB) vial was removed from -80°C and thawed at room temperature, then 50 μL was used to inoculate 50 mL seeds supplemented with 50 μg / mL kanamycin sulfate in a 250 mL baffled Erlenmeyer flask Medium (chemically defined medium). Primary seed cultures were grown for approximately 18 hours at 37°C and 250 rpm (1 inch throw). Subculture of primary seed cultures to secondary seed cultures in 500 mL baffled Erlenmeyer flasks containing 100 mL of seed medium supplemented with 50 μg / mL kanamycin sulfate, measured at 600 nm wavelength (OD600) The optical density was 0.05. Secondary seed cultures were grown at 37°C and 250 rpm (1 inch parabola) for approximately 8 hours or when an OD600 of between 2 and 4 was reached.
[0213] Sartorius Biostat B 5-L containers were filled with 2.1-L production medium (chemically defined medium) suppl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com