Preparation method of barium pyrophosphate based on barium hydrogen phosphate
A technology of barium hydrogen phosphate and barium pyrophosphate, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of no steps and lack of detailed instructions for preparation methods, and achieve the effect of improving economic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 of the present invention: a kind of preparation method of barium pyrophosphate based on barium hydrogen phosphate, comprises the following steps:
[0022] S100: Weigh 16.99g of barium carbonate, put it in a 250ml beaker, add 9.93g of 85% phosphoric acid diluted to 100ml, add while stirring, then heat and stir with a magnetic stirrer for 45min, wash 3 to 4 times after precipitation, Dry at 150°C in an oven to obtain solid barium hydrogen phosphate.
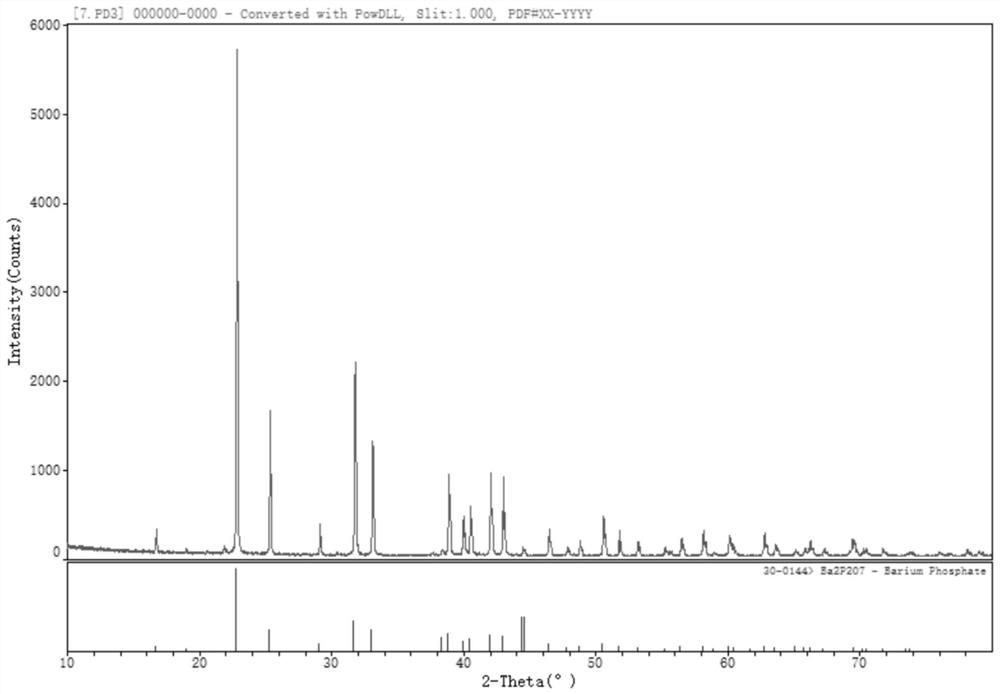
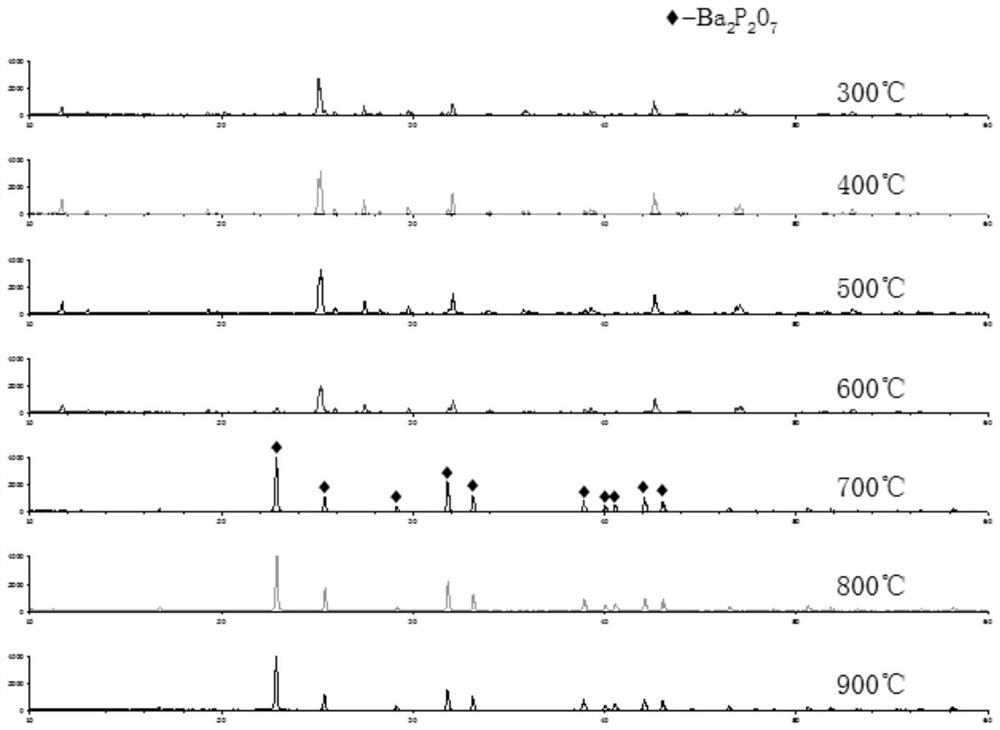
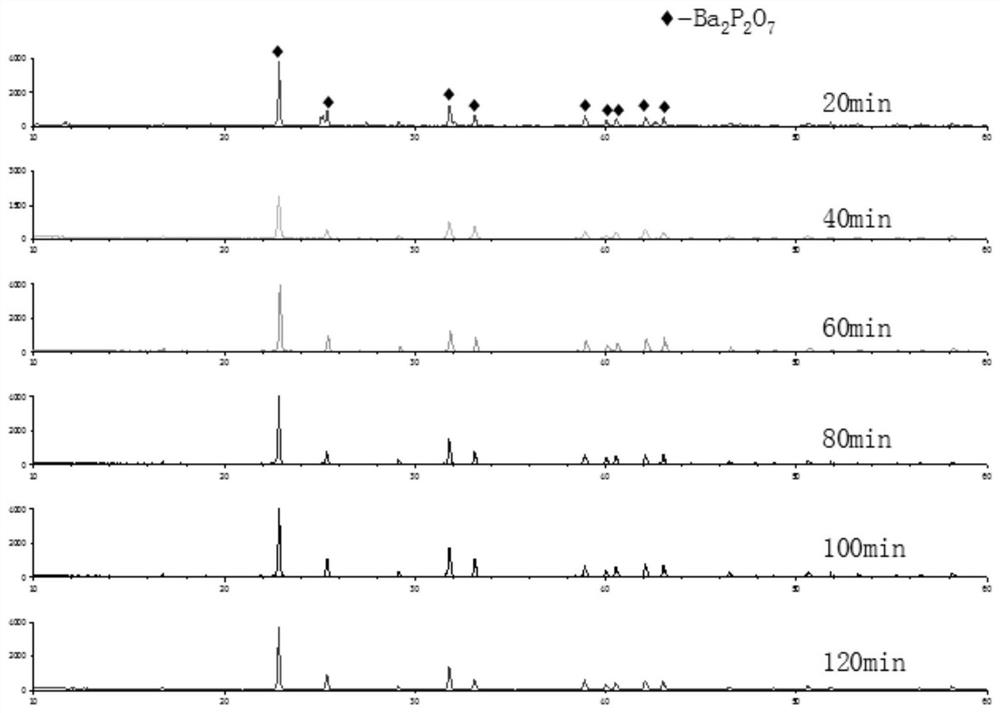
[0023] S200 : calcining the prepared barium hydrogen phosphate at 700° C. or higher for more than 20 minutes in a muffle furnace to obtain barium pyrophosphate. The barium pyrophosphate prepared by the above example has a purity of 99.1%.
Embodiment 2
[0024] Embodiment 2: a kind of preparation method of barium pyrophosphate based on barium hydrogen phosphate, comprises the following steps:
[0025] S100: Weigh 27.16g Ba(OH) 2 ·8H 2 O, 9.93g of 85% phosphoric acid, placed in a 250ml beaker, added 9.93g of 85% phosphoric acid diluted to 100ml, added while stirring, then heated and stirred with a magnetic stirrer for 45min, precipitated out, and washed the precipitate for 4-5 second, drying at 150°C in an oven to obtain solid barium hydrogen phosphate.
[0026] S200 : calcining the prepared barium hydrogen phosphate at 700° C. or higher for more than 20 minutes in a muffle furnace to obtain barium pyrophosphate. The barium pyrophosphate prepared by the above example has a purity of 99.7%.
Embodiment 3
[0027] Embodiment 3: a kind of preparation method of barium pyrophosphate based on barium hydrogen phosphate, comprises the following steps:
[0028] S100: Weighing BaCl 2H 2 O 104.69g, configured as 1L solution; weigh NaH 2 PO 4 ·2H 2 O 133.73g, configured into a 1L solution; take 50ml of each of the two solutions, mix the two solutions, add them while stirring, then heat and stir with a magnetic stirrer for 45min, and wash the precipitate for 3 to 4 times after the precipitate is precipitated, and place it in an oven. Dry at 150°C to obtain solid barium hydrogen phosphate.
[0029] S200: calcining the prepared barium hydrogen phosphate at 700° C. or higher for more than 20 minutes in a muffle furnace to obtain barium pyrophosphate. The barium pyrophosphate prepared by the above example has a purity of 99.4%.
[0030] Figure 1 to Figure 5 The order is the XRD pattern of barium pyrophosphate; the XRD pattern of calcined barium hydrogen phosphate at different temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com