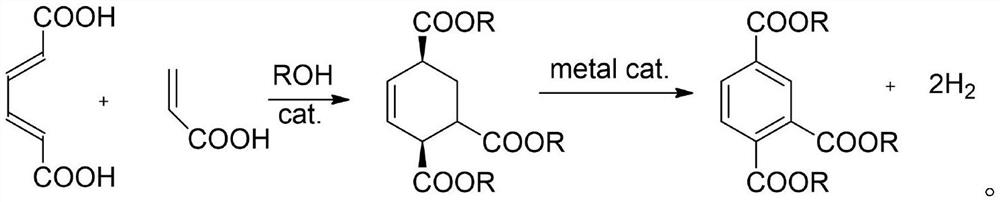
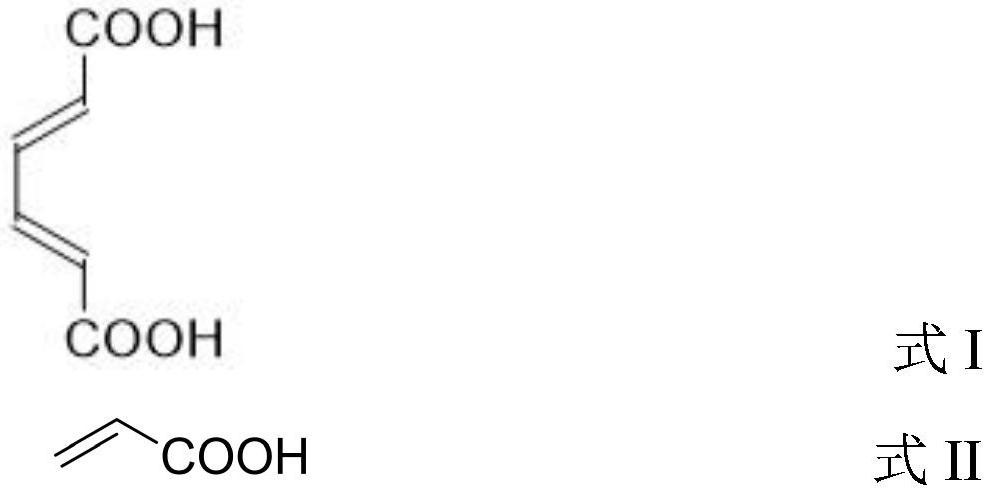Preparation method of trimellitate
A technology for trimellitic acid ester and compound is applied in the field of preparation of trimellitic acid ester, and achieves the effects of abundant raw material sources, simplified reaction steps and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1 Catalyst concentrated sulfuric acid affects esterification, Diels-Alder reaction
[0071] In this example, the effects of concentrated sulfuric acid as a catalyst on the esterification of muconic acid and acrylic acid and the Diels-Alder reaction were studied. It is specifically shown in the conversion rate of muconic acid and the yield of cyclized products.
[0072] In the reactor, add 1.7g muconic acid and 2.16g acrylic acid (muconic acid and acrylic acid molar ratio 1:3), add the concentrated sulfuric acid catalyst of 0.05g mass fraction 98% (protonic acid catalyst [H] proton amount / Muconic acid molar ratio, 10%), methanol as the reaction medium (muconic acid mass / methanol mass, 1 / 7). After stirring and mixing evenly at room temperature, the temperature was raised to 200° C. by electric heating, the reaction pressure was self-pressure, and the reaction was performed for 10 hours under magnetic stirring at 800 rpm. See Example 1 of Table 1 for the resu...
Embodiment 2
[0078] Embodiment 2 Catalyst silicotungstic acid affects esterification, Diels-Alder reaction
[0079] This example studies the effect of silicotungstic acid as a catalyst on the esterification of muconic acid and acrylic acid and the Diels-Alder reaction. It is specifically shown in the conversion rate of muconic acid and the yield of cyclized products.
[0080] The amount of reactant and the reaction conditions are the same as in Example 1, and the catalyst is changed into 0.17g silicotungstic acid (proton acid catalyst [H] proton amount / muconic acid molar ratio, 10%), and silicotungstic acid is used as a catalyst for the Diels-Alder reaction The results of the impact are shown in Example 2 of Table 1.
Embodiment 3
[0081] Embodiment 3 Catalyst SnCl 4 Effect on esterification and Diels-Alder reaction
[0082] This example studies the SnCl 4 Effect as a catalyst on the esterification of muconic acid with acrylic acid, the Diels-Alder reaction. It is specifically shown in the conversion rate of muconic acid and the yield of cyclized products.
[0083] Reactant charging amount and reaction condition are the same as embodiment 1, and catalyst changes into 0.72gSnCl 4 (metal salt catalyst / muconic acid mass ratio, 10wt%), SnCl 4 See Example 3 of Table 1 as a result of the effect of the catalyst on the Diels-Alder reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com