PEM electrolytic water hydrogen evolution catalyst with low precious metal content as well as preparation method and application of PEM electrolytic water hydrogen evolution catalyst
A catalyst and precious metal technology, applied in the application field of PEM electrolyzed water, can solve the problems of high cost and scarcity of precious metal materials hindering large-scale practical application, and achieve the effects of optimizing the distribution of internal electronic states, reducing costs, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Weigh 1g of conductive carbon and 2g of melamine mixed ball mill, the ball milling time is 1h, and the ball milling speed is 300rpm. The resulting mixture was placed in an ark and calcined at 700°C for 1 h in a tube furnace under an argon atmosphere. The calcined conductive carbon was mixed with 50 mg of platinum acetylacetonate for ball milling, the ball milling time was 1 h, and the ball milling rate was 300 rpm. The obtained ball milling product was placed in an ark, and calcined at 700 °C for 1 h in a tube furnace under an argon atmosphere to obtain nitrogen-doped Conductive carbon-supported platinum nanoparticle catalyst samples.
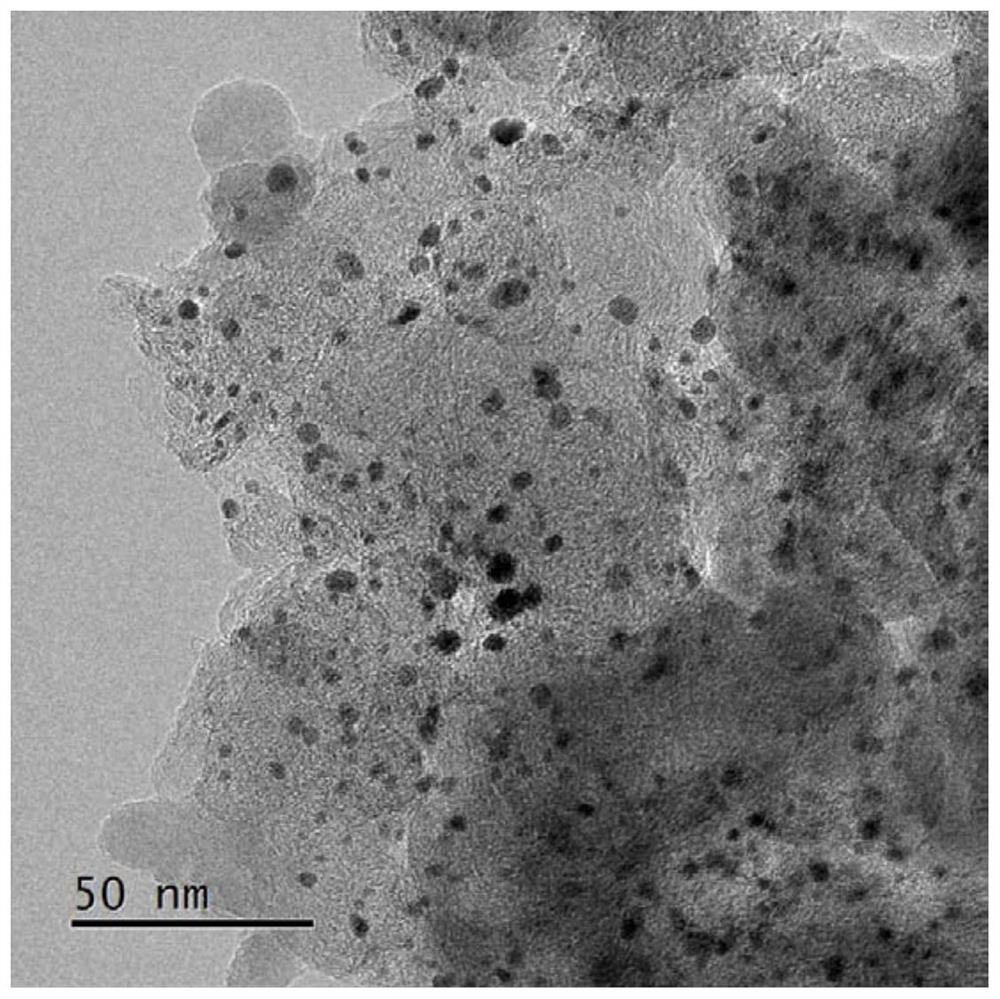
[0038] figure 2 In order to obtain the TEM image of nitrogen-doped conductive carbon-supported platinum nanoparticle catalyst, the figure 2 It can be seen that the distribution of Pt particles is relatively uniform, and the size is relatively consistent, about 5 nm.
[0039] The electrochemical activity and stability data of the nit...
Embodiment 2
[0041] 1g of conductive carbon and 2g of sodium hypophosphite were weighed and placed in different arks, the ark was placed in a tube furnace, and the sodium hypophosphite was placed upstream of the conductive carbon, and calcined at 350°C for 2h in an argon atmosphere. The calcined modified conductive carbon was mixed with 50 mg of platinum acetylacetonate for ball milling, the ball milling time was 1 h, and the ball milling speed was 300 rpm. The obtained ball-milled product was placed in an ark, and calcined at 700 °C for 1 h in a tube furnace under an argon atmosphere to obtain a phosphorus-doped conductive carbon-supported platinum nanoparticle catalyst sample.
[0042] The electrochemical activity and stability data of the phosphorus-doped conductive carbon-supported platinum nanoparticle catalyst material obtained in this example are shown in Table 1 and Table 2, respectively.
Embodiment 3
[0044] Weigh 1g of conductive carbon and 2g of melamine phosphate mixed ball milling, the ball milling time is 1h, and the ball milling speed is 300rpm. The resulting mixture was placed in an ark and calcined at 700°C for 1 h in a tube furnace under an argon atmosphere. The calcined modified conductive carbon was mixed with 50 mg of platinum acetylacetonate for ball milling, the ball milling time was 1 h, and the ball milling speed was 300 rpm. The obtained ball-milled product was placed in an ark and calcined at 600 °C for 1 h in a tube furnace under an argon atmosphere to obtain a nitrogen and phosphorus double-doped conductive carbon-supported platinum nanoparticle catalyst sample.
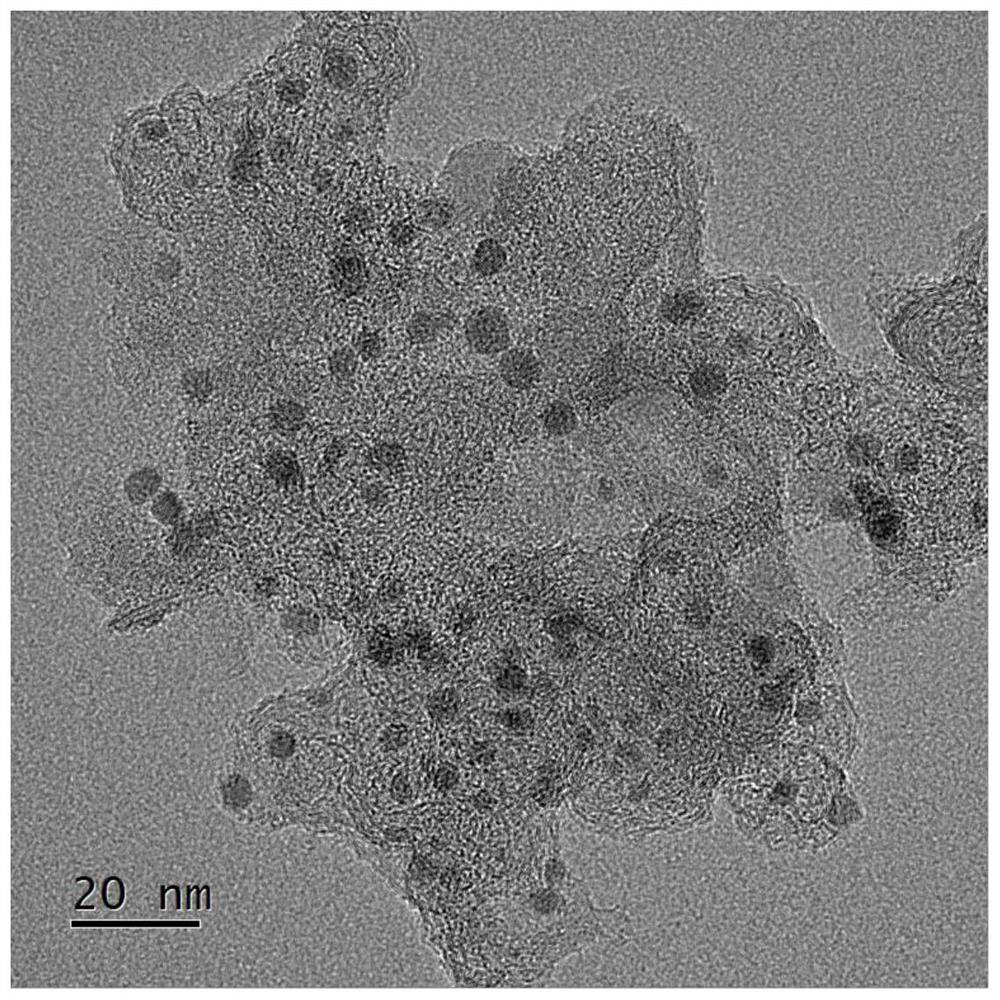
[0045] image 3 In order to obtain the TEM image of nitrogen and phosphorus double-doped conductive carbon-supported platinum nanoparticle catalyst, the image 3 It can be seen that the distribution of Pt nanoparticles is relatively uniform, and the size distribution is uniform, about 2nm.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com