Application of compound in preparation of medicine for treating Alport syndrome
A compound and syndrome technology, applied in the field of medicine, can solve the problems of reducing urinary protein excretion, unable to change the final outcome of kidney disease, etc., and achieve the effects of reducing urinary protein excretion, lowering serum creatinine level, and prolonging survival time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
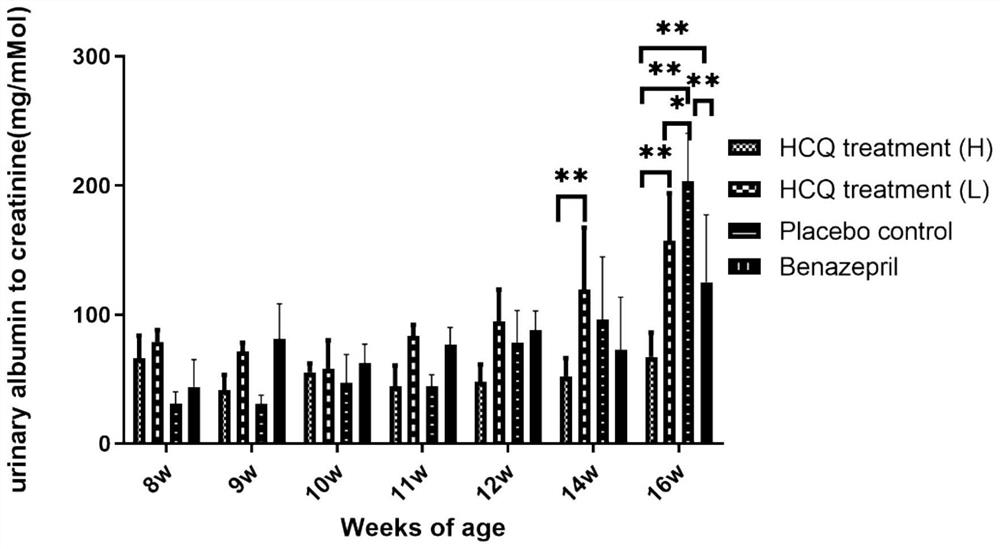
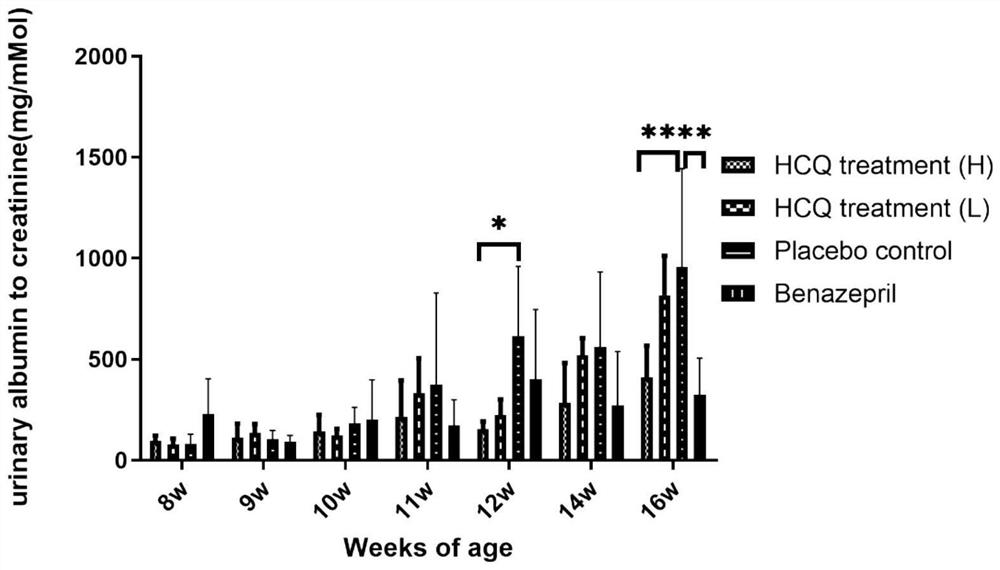
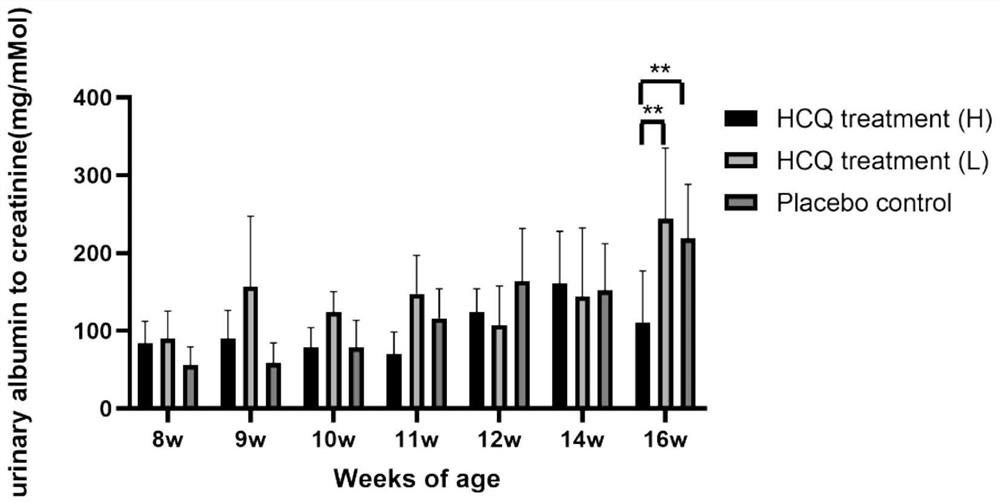
[0066] Example 1 Efficacy of HCQ in the treatment of X-linked Alport syndrome (XLAS) mice
[0067] The experiment set up 2 mouse models of X-linked Alport syndrome (XLAS). The first XLAS mouse model is the classical mouse model (COL4A5 p.G5X), purchased from Jackson Laboratory (JAX STOCK#3376), genetic background: C57BL / 6J; gene name: COL4A5; mutation type: nonsense mutation ( c.G213T, p.Gly5X), genotype: X G5X / X w (female heterozygote), X G5X / X G5X or X G5X / Y (male hemizygote) has been widely used in many studies at home and abroad. The second XLAS mouse model is the model mouse constructed as described in the first part of the study, referring to the suspected pathogenic gene variant found in the clinic and using CRISPR / Cas9 gene editing technology to construct a new frameshift mutation mouse model of COL4A5, and complete the phenotype Evaluate. Genetic background: C57BL / 6; Gene name: COL4A5; Mutation type: Frameshift mutation (c.2440delG, p.Gly814fs); Genotype: X ...
Embodiment 2
[0084] Example 2 HCQ treats children with X-linked Alport syndrome (XLAS)
[0085] This study is a single-center, concurrent, non-randomized, comparative study to evaluate the efficacy and safety of hydroxychloroquine combined with benazepril hydrochloride tablets and benazepril hydrochloride tablets alone (current first-line treatment regimen) in the treatment of children with AS sex. Subjects were included in the study group in a 1:1 ratio.
[0086] This clinical study was approved by the ethics committee of our hospital (ethics batch number: 2019R063-E02), and all the children included in this clinical study and their legal guardians signed the informed consent.
[0087] Drug usage and dosage:
[0088] HCQ treatment group: basic treatment combined with hydroxychloroquine sulfate treatment. The details are as follows: Hydroxychloroquine Sulfate Tablets (6.5mg / kg / day, taken in divided doses) + Benazepril Hydrochloride Tablets (5-10mg / time, 1 time / day), orally 15-30 minutes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com