Leonurine, polygonatum polysaccharide and deoxynojirimycin pharmaceutical composition and application thereof
A technology of deoxynojirimycin and polysaccharide polysaccharide is applied in the directions of drug combination, active ingredients of heterocyclic compounds, and pharmaceutical formulations, which can solve problems such as many adverse reactions, and achieve the effects of less toxic side effects, high content of active ingredients, and improving blood clots.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation method of the pharmaceutical composition of leonurine, polysaccharide polysaccharide and deoxynojirimycin of the present invention is as follows: weigh 75 g of leonurine, 50 g of polysaccharide polysaccharide, 500 g of deoxynojirimycin, 290 g of starch, 45 g of maltodextrin, and 40 g of mannitol. Mix well with a 100-mesh sieve, add an appropriate amount of distilled water to make soft materials, pass through a 18-mesh sieve for granulation, dry at 45-60°C and pass through a 16-mesh sieve to granulate, fill the capsules to make capsules (1g / capsule), or press into tablets It can be made into tablets (1 g / tablet), or filled into granules (10 g / pack), in the form of granules or tablets or capsules.
Embodiment 2
[0038] The preparation method of the pharmaceutical composition of leonurine, polysaccharide polysaccharide and deoxynojirimycin of the present invention is as follows: weigh 93.75 g of leonurine, 50 g of polysaccharide polysaccharide, 625 g of deoxynojirimycin, 175 g of starch, 30 g of maltodextrin, and 26.25 g of mannitol , pass through a 100-mesh sieve and mix well, add an appropriate amount of distilled water to make soft materials, pass through a 18-mesh sieve for granulation, dry at 45-60°C and pass through a 16-mesh sieve to granulate, fill the capsules to make capsules (1g / capsule), or Tablets are made into tablets (1 g / tablet), or filled into granules (10 g / pack), and the formulations are in the form of granules or tablets or capsules.
Embodiment 3
[0040] The preparation method of the pharmaceutical composition of leonurine, polysaccharide polysaccharide and deoxynojirimycin of the present invention is as follows: weighing 90 g of leonurine, 45 g of polysaccharide polysaccharide, 720 g of deoxynojirimycin, 90 g of starch, 30 g of maltodextrin, and 20 g of mannitol, Mix well with a 100-mesh sieve, add an appropriate amount of distilled water to make soft materials, pass through a 18-mesh sieve for granulation, dry at 45-60°C and pass through a 16-mesh sieve to granulate, fill the capsules to make capsules (1g / capsule), or press into tablets It can be made into tablets (1 g / tablet), or filled into granules (10 g / pack), in the form of granules or tablets or capsules.
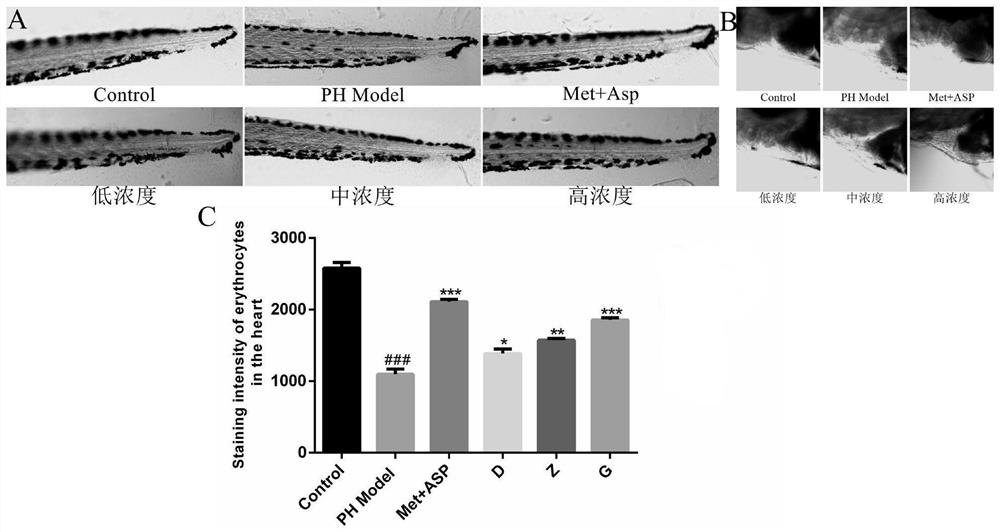
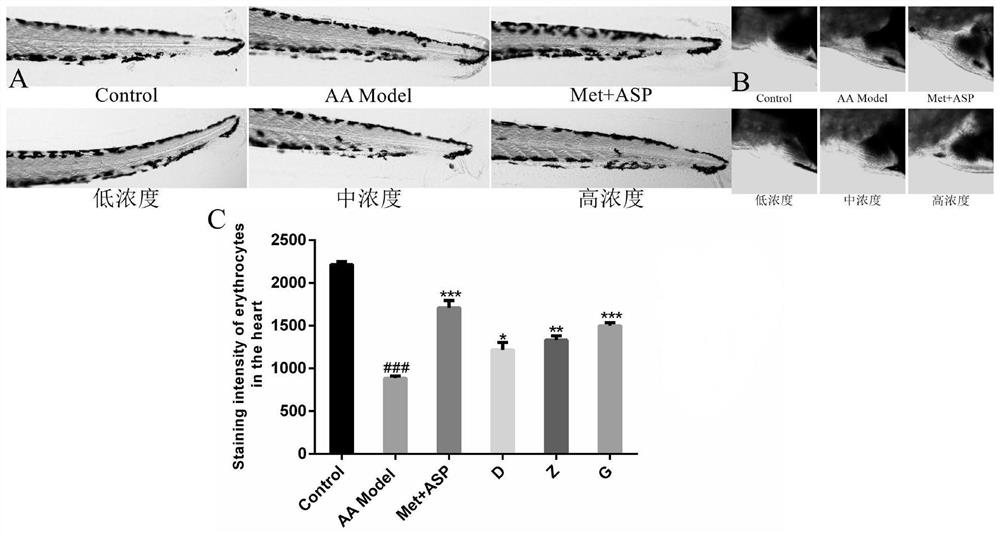
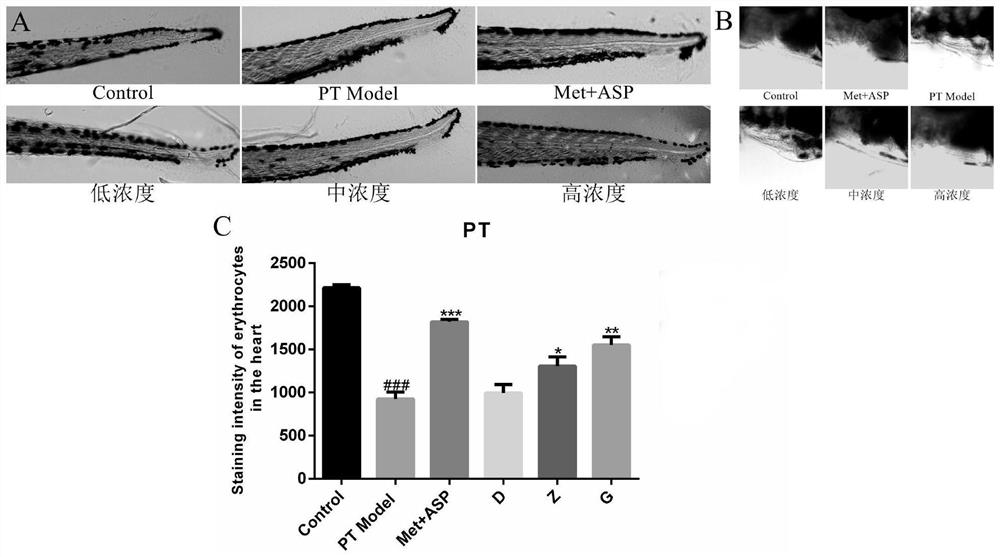
[0041] The pharmaceutical composition of leonurine, polysaccharide polysaccharide and deoxynojirimycin has the effect of treating diabetes, lowering blood sugar and improving thrombus. These pharmacological effects are confirmed by the following pharmacodynami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com