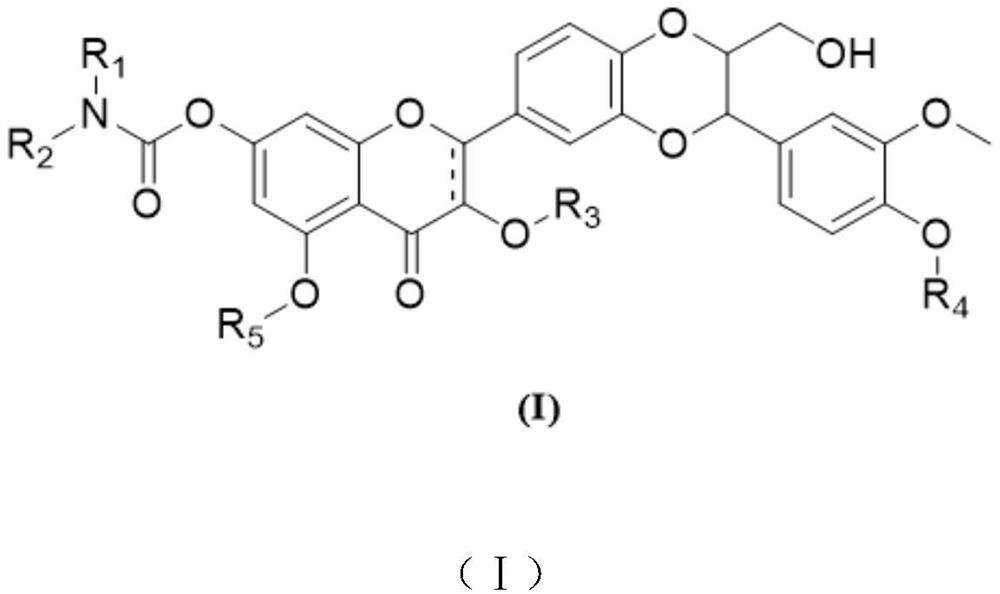
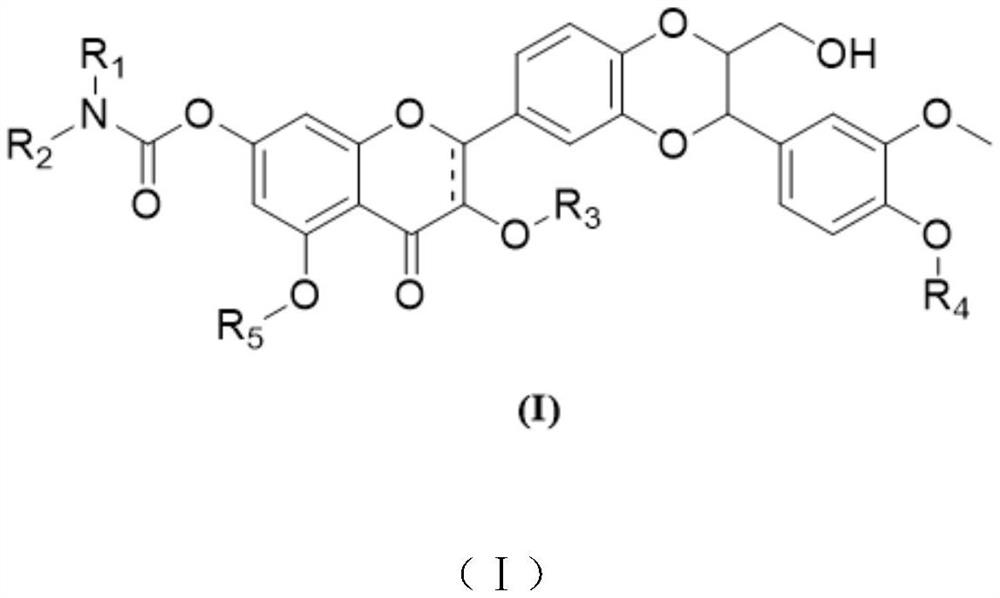
Silybin derivative containing carbamic acid structure and application of silybin derivative
A technology of silibinin and carbamate, applied in the field of medicine, can solve the problems of limiting the anti-tumor application of silibinin, poor water solubility and fat solubility of silibinin, and less than 5% bioavailability, etc. Social and economic benefits, low cost and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of Compound 1
[0034]
[0035] At room temperature, silibinin (465.9 mg, 0.97 mmol, 1.0 eq) and tetrahydrofuran (7.0 mL, 15.0 vol) were added to a 5 mL three-necked round-bottom flask, and the argon was replaced 6 times. The stirring was turned on, and the system was cooled to 0 -5°C, N,N-diisopropylethylamine (249.6mg, 1.93mmol, 2.0eq) and 4-dimethylaminopyridine (23.6mg, 0.19mmol, 0.2eq) were added to the reaction flask, and argon was replaced 6 times, [1,4']bipiperidine-1'-formyl chloride (234.0 mg, 1.01 mmol, 1.05 eq) was added to the reaction flask, argon was replaced 6 times, and the temperature of the system was controlled at 0-5 °C to stir the reaction 24 hours. After the reaction was detected by TLC, 30 mL of water and 30 mL of ethyl acetate were added to the system, stirred for 10 min, and allowed to stand for 10 min. All the organic phases were washed by adding 50 mL of water, the organic phase was separated, 50 mL of brine was added to wash, ...
Embodiment 2
[0038] Synthesis of Compound 2
[0039]
[0040] At room temperature, silibinin (241.2 mg, 0.50 mmol, 1.0 eq) and tetrahydrofuran (3.6 mL, 15.0 vol) were added to a 5 mL three-necked round-bottomed flask, and the argon was replaced 6 times. The stirring was turned on, and the system was cooled to 0 -5°C, N,N-diisopropylethylamine (193.8mg, 1.50mmol, 3.0eq) and 4-dimethylaminopyridine (18.3mg, 0.15mmol, 0.3eq) were added to the reaction flask, and argon was replaced 6 times, diisopropylcarbamoyl chloride (163.7 mg, 1.00 mmol, 2.0 eq) was added to the reaction flask, argon was replaced 6 times, and the temperature of the system was controlled at 0-5 °C and stirred for 24 hours. After TLC detected the reaction, add 20 mL of water and 20 mL of ethyl acetate to the system, stir for 10 min, let stand for 10 min, separate the organic phase for temporary storage, add 20 mL of ethyl acetate to the aqueous phase, stir for 10 min, let stand for 10 min, separate and combine All organi...
Embodiment 3
[0043] Synthesis of Compound 3
[0044]
[0045] At room temperature, silibinin (241.2 mg, 0.50 mmol, 1.0 eq) and tetrahydrofuran (3.6 mL, 15.0 vol) were added to a 5 mL three-necked round-bottomed flask, and the argon was replaced 6 times. The stirring was turned on, and the system was cooled to 0 -5°C, N,N-diisopropylethylamine (129.2mg, 1.0mmol, 2.0eq) and 4-dimethylaminopyridine (12.2mg, 0.10mmol, 0.2eq) were added to the reaction flask, and argon was replaced 6 times, diethylcarbamoyl chloride (71.2 mg, 0.53 mmol, 1.05 eq) was added to the reaction flask, argon was replaced 6 times, and the temperature of the system was controlled at 0-5 °C and stirred for 24 hours. After TLC detected the reaction, add 20 mL of water and 20 mL of ethyl acetate to the system, stir for 10 min, let stand for 10 min, separate the organic phase for temporary storage, add 20 mL of ethyl acetate to the aqueous phase, stir for 10 min, let stand for 10 min, separate and combine All organic pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com