Method for detecting whether liquid adding amount of immunoturbidimetric reagent is abnormal or not
An immunoturbidimetric and detection method technology, applied in the field of biochemical detection, which can solve the problems of reporting wrong test results, abnormal amount of added reagents, misdiagnosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 measurement results:
[0050] ①The target item to be tested: β2 microglobulin;
[0051] ②Experimental material: The sample to be tested is a fresh human urine sample;
[0052] ③Reagent1: 4-hydroxyethylpiperazine ethanesulfonic acid buffer 50mM~500mM, Reagent 1 is prefilled in the colorimetric chamber;
[0053] ④Reagent2: 4-hydroxyethylpiperazine ethanesulfonic acid buffer 50mM~500mM; β2 microglobulin antibody coating latex 0.1%~0.5%; trehalose 1%~10%;
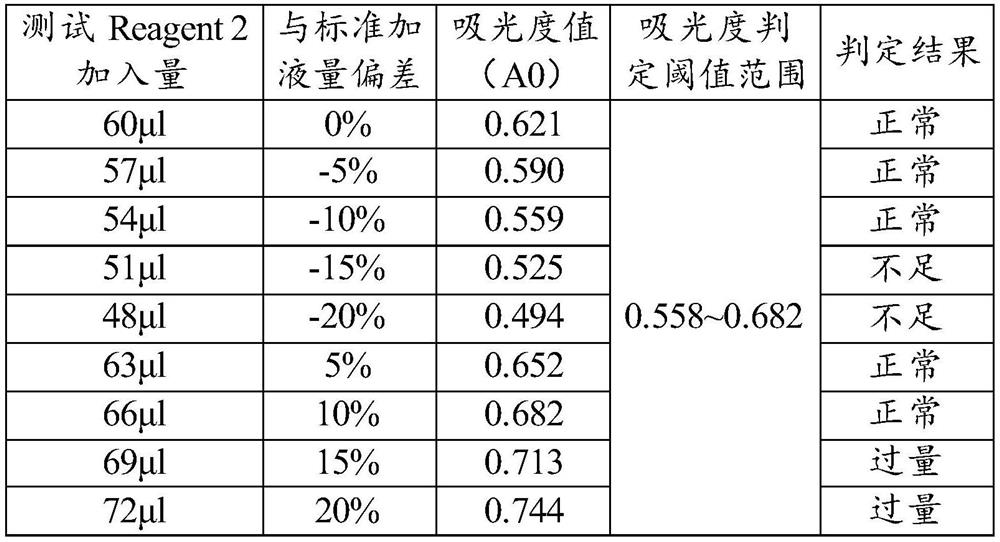
[0054] ⑤ Absorbance judgment threshold: 0.558~0.682;
[0055] ⑥Testing process: Pre-fill 240μl of Reagent 1 in the reaction chamber, transfer 60μl of Reagent 2 to the aforementioned reaction chamber with the reagent / sample needle during the test, mix well and read the absorbance A0. Compare A0 with the set threshold range of the project, if it is higher than 0.682, it indicates that Reagent2 is over-added, and if it is lower than 0.558, it indicates that Reagent 2 is under-added. Reagent / sample needle Trans...
Embodiment 2
[0059] ① Target items to be tested: C-reactive protein;
[0060] ②Experimental materials: The samples to be tested are fresh human serum samples;
[0061] ③Reagent1: 4-Hydroxyethylpiperazine ethanesulfonic acid buffer 50mM~500mM, NaCl 150Mm~500mM; Reagent 1 is prefilled in the colorimetric chamber;
[0062] ④Reagent2: 4-hydroxyethylpiperazine ethanesulfonic acid buffer 50mM~500mM; C-reactive protein antibody coating latex 0.1%~0.5%; sucrose 1%~10%;
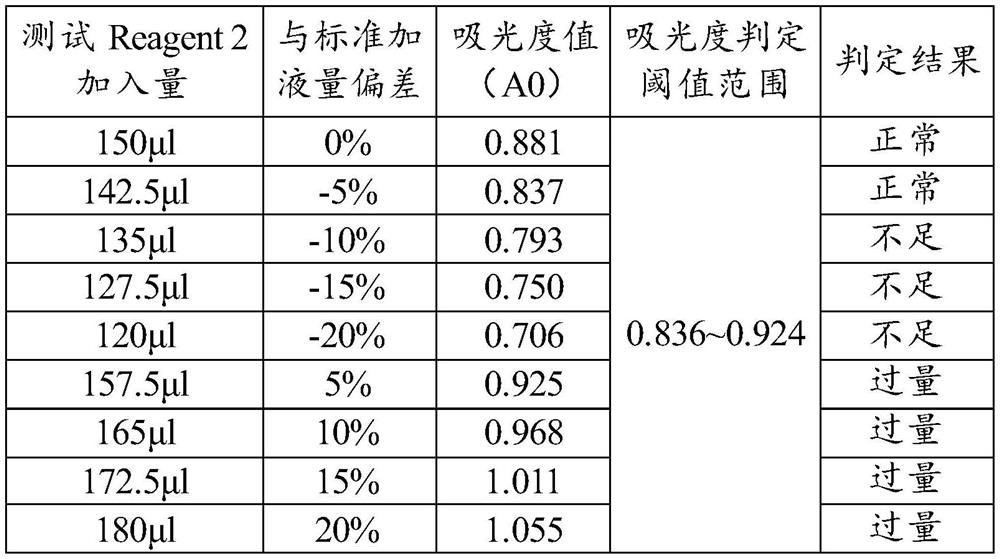
[0063] ⑤ Absorbance judgment threshold: 0.836~0.924;
[0064] ⑥Testing process: Pre-fill 150μl Reagent 1 in the reaction chamber, transfer 150μl Reagent 2 to the aforementioned reaction chamber with the reagent / sample needle during the test, mix well and read the absorbance A0. Compare A0 with the set threshold range of the project, if it is higher than 0.924, it indicates that Reagent 2 is over-added, and if it is lower than 0.836, it indicates that Reagent 2 is under-added. Reagent / sample needle Transfer 3 μl of the serum sam...
Embodiment 3
[0068] ①The target item to be tested: myoglobin;
[0069] ②Experimental materials: The samples to be tested are fresh human serum samples;
[0070] ③Reagent1: 4-hydroxyethylpiperazine ethanesulfonic acid buffer 50mM~500mM, NaCl150Mm~500mM, polyethylene glycol 6000 1.0%~5.0%; Reagent 1 is prefilled in the colorimetric chamber;
[0071] ④Reagent2: 4-hydroxyethylpiperazine ethanesulfonic acid buffer 50mM~500mM; myoglobin antibody coating latex 0.1%~0.5%; sucrose 1%~10%;
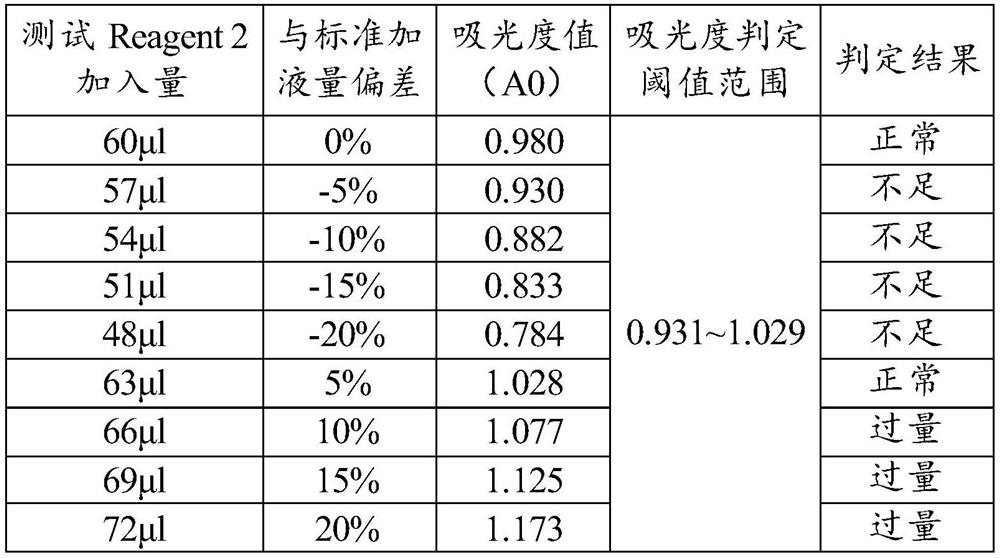
[0072] ⑤ Absorbance judgment threshold: 0.931~1.029;
[0073] ⑥ Detection process: Pre-fill 180 μl of Reagent 1 in the reaction chamber, transfer 60 μl of Reagent 2 to the aforementioned reaction chamber with the reagent / sample needle during the test, mix well and read the absorbance A0. Compare A0 with the set threshold range of the project, if it is higher than 1.029, it indicates that Reagent 2 is over-added, and if it is lower than 0.931, it indicates that Reagent 2 is under-added. Reagent / sample needle T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com