Crystal form of neurokinin-1 antagonist and preparation method thereof
A technology of crystal form and solvent, applied in the field of crystal form of neurokinin-1 antagonist, can solve the problems of difficult filtration, poor product stability, easy agglomeration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Preparation of compound of formula II
[0070]
[0071] first step:
[0072]
[0073] in N 2 Under protection, compound 1 (2.43g, 4.86mmol, 1eq) was weighed in a 100mL three-necked flask, dissolved in dichloromethane (36mL), added diisopropylethylamine (5g, 38.76mmol, 8eq), cooled to - At 30° C., trimethylchlorosilane (1.36 g, 12.52 mmol, 2.6 eq) was added, and the mixture was stirred at room temperature for 2 h. Cool to -25°C, add dropwise a solution of chloromethyl chloroformate (0.77g, 6mmol, 1.23eq) in dichloromethane, control the temperature at -20°C to -5°C and stir until the reaction is complete, pour the reaction solution into ice water , separation, extraction with dichloromethane, adding water and 1N hydrochloric acid solution, separation, washing with brine, saturated aqueous sodium bicarbonate solution and brine successively, drying over anhydrous sodium sulfate, filtration, and concentration to obtain 3.0 g of yellow gum , the yield was ...
Embodiment 2
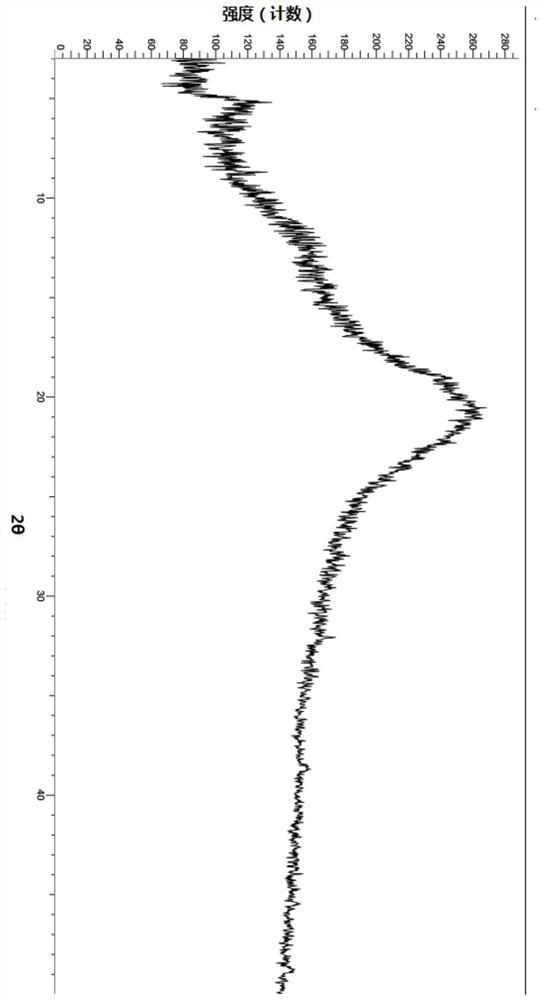
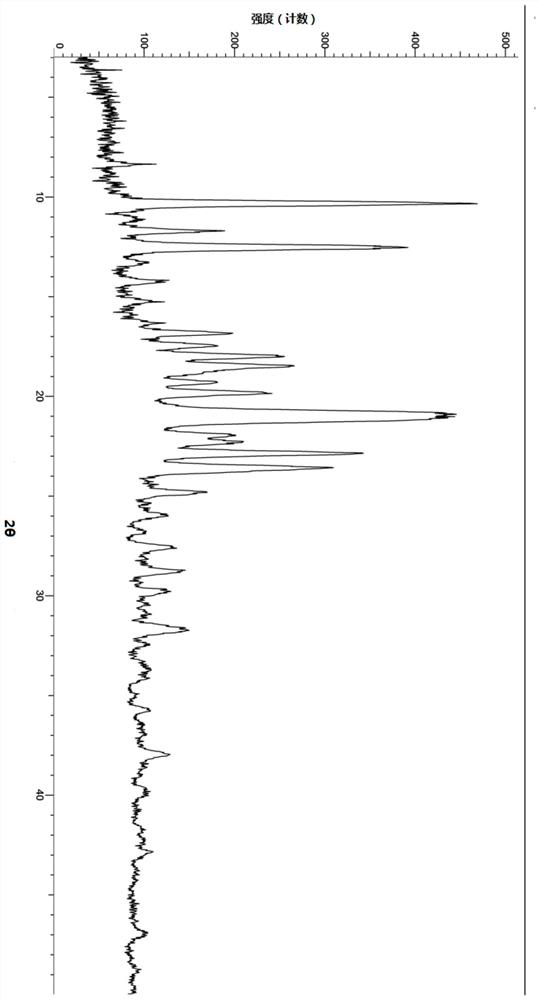
[0119] Example 2: Preparation of Crystal Form of Compound A of Formula II
[0120] About 100 mg of the compound shown in formula II was weighed, added to 1 mL of diethyl ether, stirred at room temperature to dissolve the clear liquid, slurried to separate out a solid, and vacuum-dried to obtain the crystal form A of the compound shown in formula II after centrifugation, and its characteristic peak positions are shown in Table 3:
[0121] Table 3: XRD characteristic peak positions of crystal form A
[0122]
[0123]
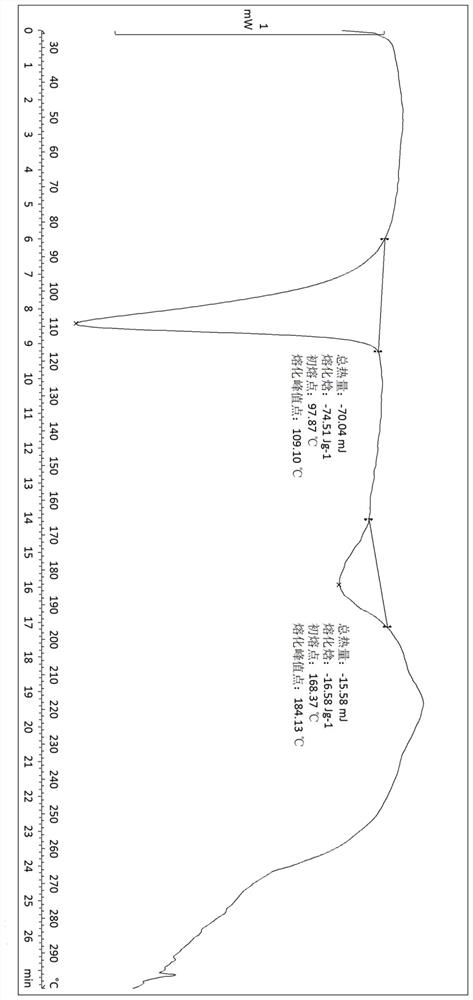
Embodiment 3
[0124] Example 3: Stability investigation of crystal form A
[0125] The crystal form of compound A of formula II was placed under the condition of 4°C to investigate its stability.
[0126] Table 4
[0127]
[0128] The results showed that the crystal form A had good physical and chemical stability under the stability condition of 4 ℃ for 3 months.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com