Chiral covalent organic framework material, preparation method and application
A covalent organic framework and chirality technology, applied in the field of chiral covalent organic framework materials, can solve the problems of low modification efficiency, complex synthesis of chiral monomers, lack of universality of chiral induction strategies, etc. Selective, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
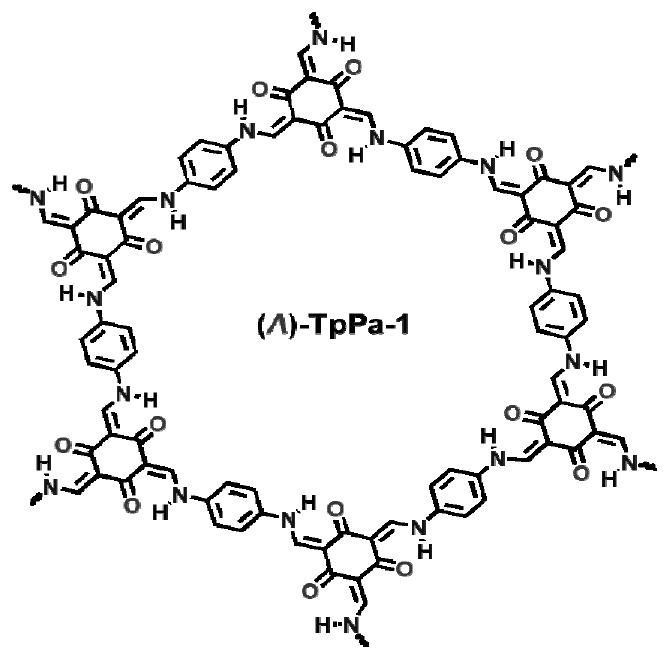
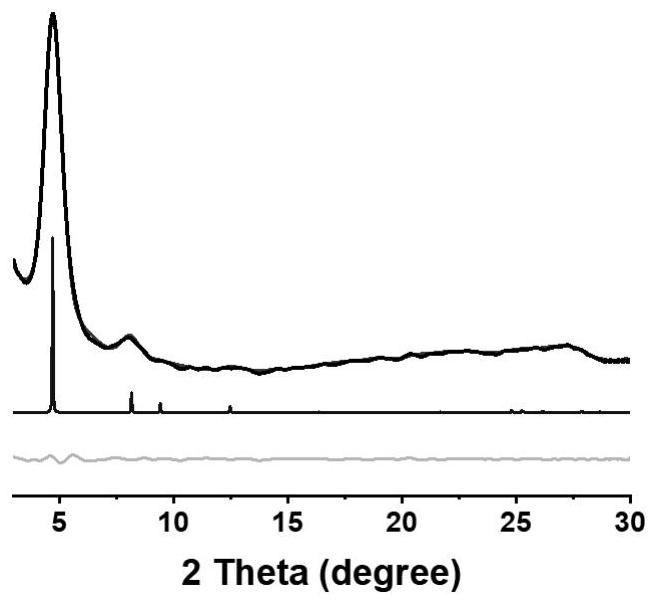
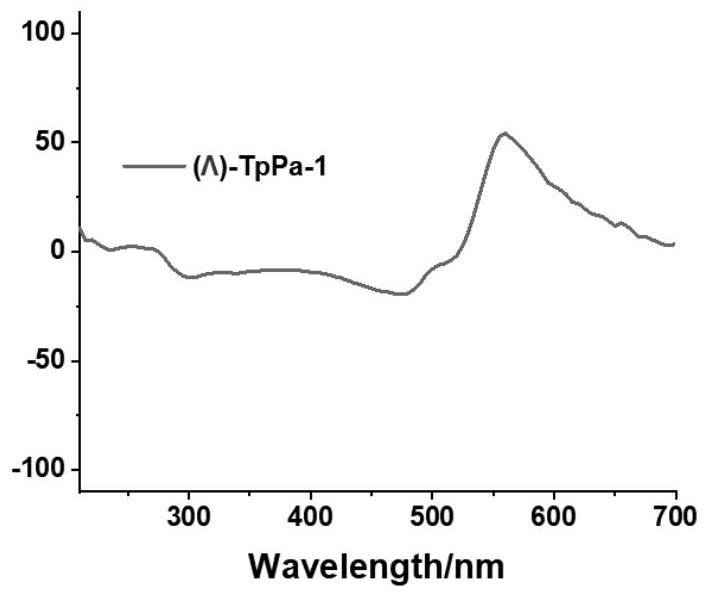
[0049] Example 1: This example provides a method for preparing a chiral covalent organic framework material (Λ)-TpPa-1, the specific steps are as follows, 1,3,5-triformyl-2,4,6-m-benzenetri Phenol (63.0 mg, 0.30 mmol), p-phenylenediamine (48.6 mg, 0.45 mmol), S-2-methylpyrrolidine (7.7 mg, 0.09 mmol), dissolved in dichloromethane (6.0 mL), and reacted at room temperature 7 days. After the reaction, centrifuged to obtain brick red powder, washed with dichloromethane until the clear liquid was colorless, then washed once with ethanol, acetone, and ether in turn, and vacuum-dried at 60 ° C for 12 hours to obtain a chiral covalent organic framework material ( Δ)-TpPa-1. The structural formula of embodiment 1 sees figure 1 , powder diffraction PXRD see figure 2 , see circular dichroism CD spectrum image 3 , see SEM Figure 4 , see the infrared spectrum Figure 5 , see macro photo Image 6 .
Embodiment 2
[0050] Embodiment 2: This embodiment provides a method for preparing a chiral covalent organic framework material (Δ)-TpPa-1, the specific steps are as follows, 1,3,5-triformyl-2,4,6-m-benzenetri Phenol (63.0mg, 0.30mmol), p-phenylenediamine (48.6mg, 0.45mmol), R-2-methylpyrrolidine (7.7mg, 0.09mmol), dissolved and dispersed in dichloromethane (6.0mL), reacted at room temperature 7 days. After the reaction, centrifuged to obtain brick red powder, washed with dichloromethane until the clear liquid was colorless, then washed once with ethanol, acetone, and ether in turn, and vacuum-dried at 60 ° C for 12 hours to obtain a chiral covalent organic framework material ( Δ)-TpPa-1. The structural formula of embodiment 2 sees Figure 7 , powder diffraction PXRD see Figure 8 , see circular dichroism CD spectrum Figure 9 , see SEM Figure 10 , see the infrared spectrum Figure 11 , see macro photo Figure 12 .
Embodiment 3
[0051] Embodiment 3: This embodiment provides a method for preparing a chiral covalent organic framework material (Λ)-TpPa-2, the specific steps are as follows, 1,3,5-triformyl-2,4,6-m-benzenetri Phenol (63.0 mg, 0.30 mmol), p-phenylenediamine (61.0 mg, 0.45 mmol), S-2-methylpyrrolidine (7.7 mg, 0.09 mmol), dissolved in dichloromethane (6.0 mL), and reacted at room temperature 7 days. After the reaction, centrifuged to obtain brick red powder, washed with dichloromethane until the clear liquid was colorless, then washed once with ethanol, acetone, and ether in turn, and vacuum-dried at 60 ° C for 12 hours to obtain a chiral covalent organic framework material ( Δ)-TpPa-2. The structural formula of embodiment 3 sees Figure 13 , powder diffraction PXRD see Figure 14 , see circular dichroism CD spectrum Figure 15 , see SEM Figure 16 , see the infrared spectrum Figure 17 , see macro photo Figure 18 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com