Preparation method of 4-chloro-3-nitroanisole
A technology of nitroanisole and nitrophenol is applied in the field of preparation of 4-chloro-3-nitroanisole, and can solve the problems of difficulty in treating cuprous chloride wastewater, large environmental pollution, potential safety hazards and the like , to achieve the effect of simple and easy operation, improved yield and high economical practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
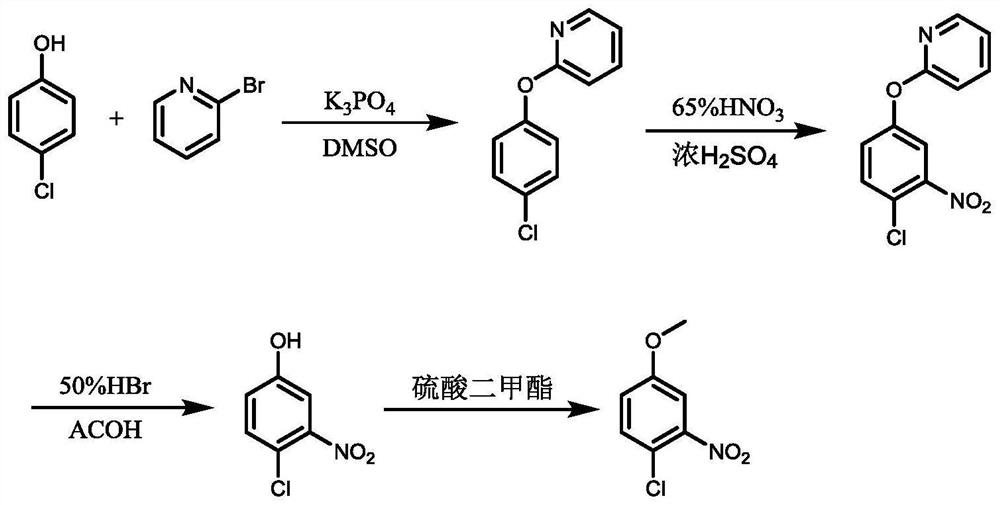
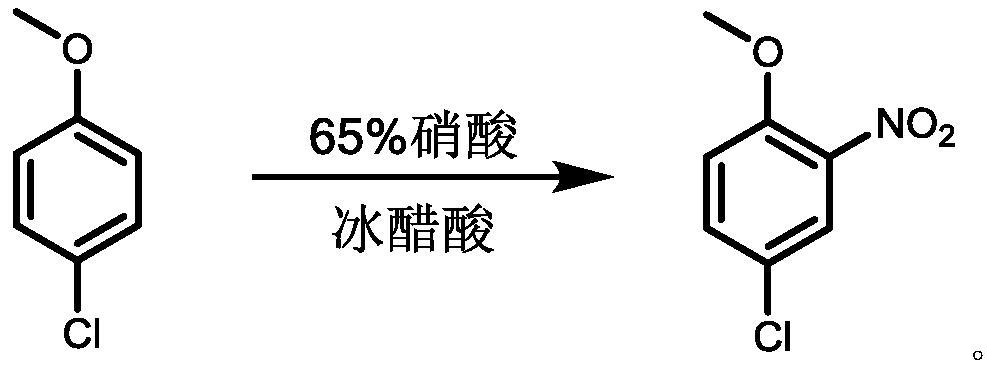
[0021] 4-Chloro-3-nitroanisole is prepared by the method of the present invention, and the reaction process is shown in the appendix figure 1 , the specific operation steps are as follows:
[0022] Step S1: put (10g, 0.0781mol) p-chlorophenol, (12.3g, 0.0781mol) 2-bromopyridine, (24.8g, 0.1171mol) potassium phosphate), 50ml dimethyl sulfoxide into a three-necked flask, stir and mix And heat up to 100 ℃, keep the reaction for 16h, after the reaction, cool down to room temperature, filter and separate out the insoluble solid, the filtrate is black and clear; the filtrate is added dropwise to 200ml of water, and no solid precipitation is observed after the dropwise addition; then, the reaction mixture is Extract with dichloromethane and concentrate the organic phase to dryness under reduced pressure to give 14.71 g of product. HPLC results showed that the percentage content of 2-(4-chlorophenoxy)pyridine in the product was 98.76%, and the molar yield was 91.9% (calculated as p-c...
Embodiment 2
[0027]Step S1: put (10g, 0.0781mol) p-chlorophenol, (12.3g, 0.0781mol) 2-bromopyridine, (16.5g, 0.0781mol) potassium phosphate), 50ml dimethyl sulfoxide into a three-necked flask, stir and mix The temperature was raised to 80°C, and the reaction was kept for 24 hours. After the reaction was completed, the temperature was lowered to normal temperature. The insoluble solid was removed by filtration and separation. Extract with dichloromethane and concentrate the organic phase to dryness under reduced pressure to give 12.34 g of product. HPLC results showed that the percentage content of 2-(4-chlorophenoxy)pyridine in the product was 98.21%, and the molar yield was 77% (calculated as p-chlorophenol).
[0028] Step S2: put (22.68g, 0.234mol) 65wt% nitric acid into the three-necked flask, freeze and cool down to 0°C, slowly add (11.47g, 0.117mol) concentrated sulfuric acid dropwise to the reaction solution, there is obvious exothermic phenomenon, add dropwise Finished adding (12g,...
Embodiment 3
[0032] Step S1: put (10g, 0.0781mol) p-chlorophenol, (24.6g, 0.1562mol) 2-bromopyridine, (49.5g, 0.2343mol) potassium phosphate), 100ml dimethyl sulfoxide into a three-necked flask, stir and mix The temperature was raised to 120°C, and the reaction was kept for 16 hours. After the reaction was completed, the temperature was lowered to room temperature. The insoluble solid was removed by filtration and separation. Extract with dichloromethane, and concentrate the organic phase to dryness under reduced pressure to obtain 15.23 g of product. HPLC results showed that the percentage content of 2-(4-chlorophenoxy)pyridine in the product was 98.21%, and the molar yield was 95% (calculated as p-chlorophenol).
[0033] Step S2: put (34.02g, 0.351mol) 65wt% nitric acid in a three-necked flask, freeze and cool down to 0°C, slowly add (22.94g, 0.234mol) concentrated sulfuric acid dropwise to the reaction solution, there is obvious exothermic phenomenon, add dropwise Finished adding (12g,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com