Felodipine propranolol hydrochloride compound preparation and preparation method thereof
A technology of dipine propranolol hydrochloride compound and preparation, which is applied in the field of felodipine propranolol hydrochloride compound preparation and its preparation, and can solve problems such as reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
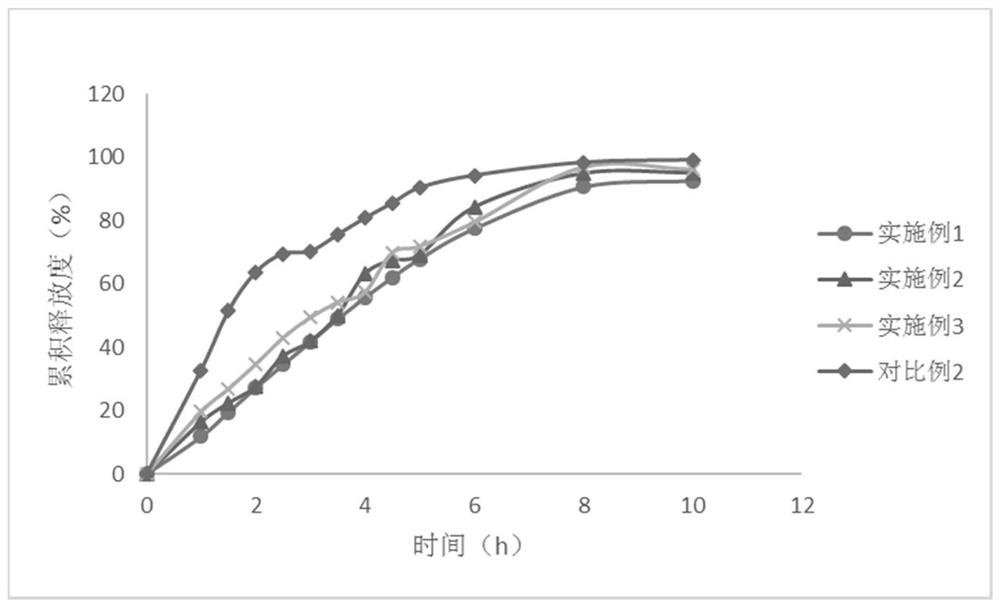
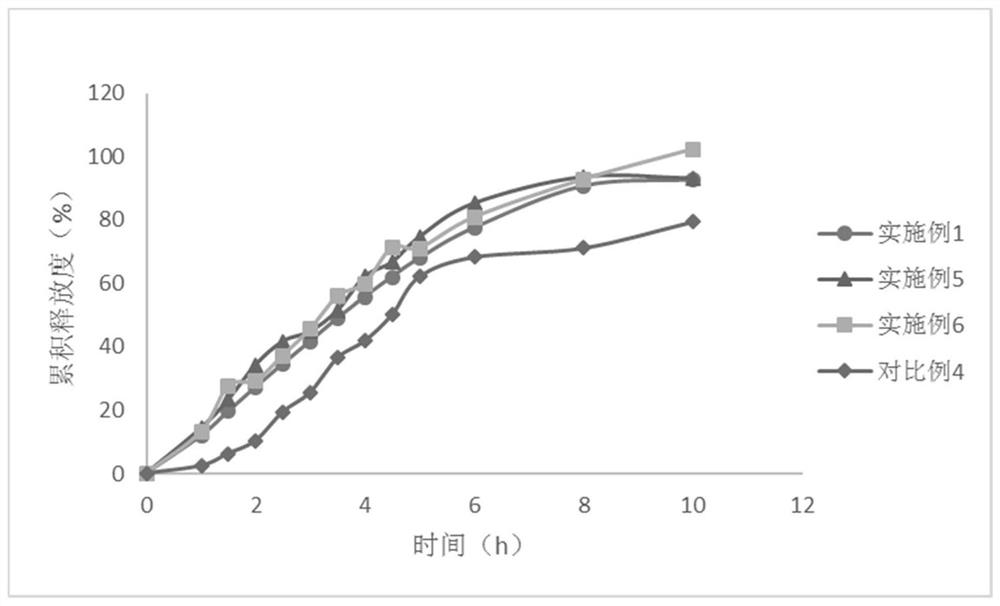
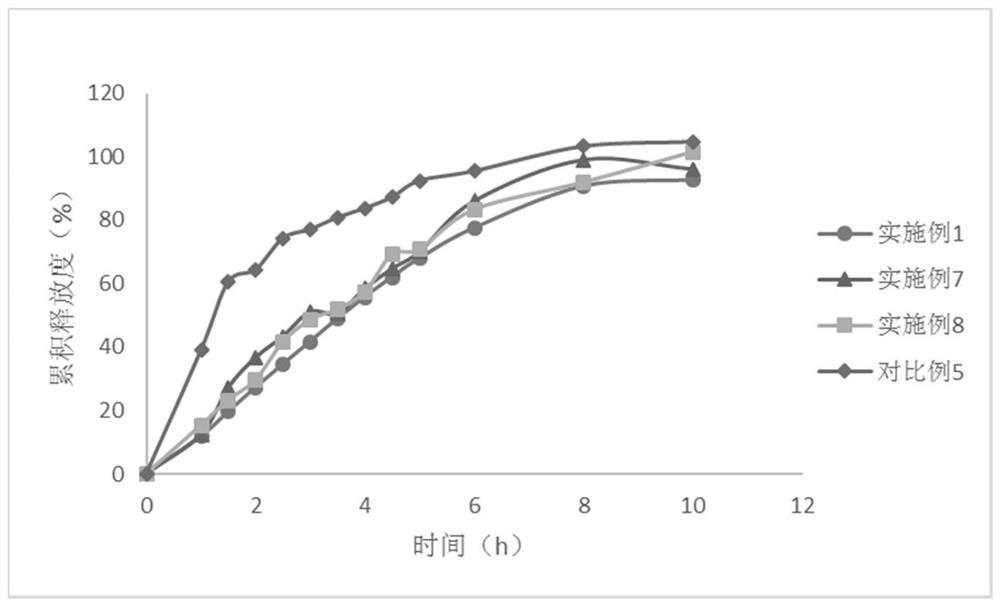
Embodiment 1
[0081] The present embodiment provides the compound preparation of felodipine and propranolol hydrochloride, and its prescription is as follows:
[0082]
[0083] Preparation:
[0084] 1) Inner layer granulation: add propranolol hydrochloride, anhydrous lactose, hydroxypropyl cellulose and silicon dioxide into the wet mixing granulator according to the granulation formula and content in the above table, stir for 10min, add purified Water granulation; granules are dried at 50°C.
[0085]2) Outer layer granulation: add granulation formula and content according to the above table, granulate hypromellose (K100M), hypromellose (E50), hypromellose, felodipine, microcrystalline cellulose, gallic Propyl acrylate, micropowder silica gel and polyoxyethylene hydrogenated castor oil are mixed evenly, then sodium stearyl fumarate is added, and granulated;
[0086] 3) Take the particles in step 1) and use a 5mm flat oblique punching sheet.
[0087] 4) Take part of the granules in step...
Embodiment 2-3
[0090] The present embodiment provides the compound preparation of felodipine and propranolol hydrochloride, and its prescription is as follows:
[0091]
[0092] Preparation:
[0093] 1) Inner layer granulation: add propranolol hydrochloride, anhydrous lactose, and silicon dioxide to the wet mixing granulator according to the granulation formula and content in the above table, stir for 10 min, and add purified water to granulate; Dry at 50°C.
[0094] 2) Outer layer granulation: add granulation formula and content according to the above table, granulate hypromellose (K100M), hypromellose (E50), hypromellose, felodipine, microcrystalline cellulose, gallic Propyl acrylate, micropowder silica gel and polyoxyethylene hydrogenated castor oil are mixed evenly, then sodium stearyl fumarate is added, and granulated;
[0095] 3) Take the particles in step 1) and use a 5mm flat oblique punching sheet.
[0096] 4) Take part of the granules in step 2) and fill it into the die, add ...
Embodiment 4
[0099] The compound preparation of felodipine and propranolol hydrochloride, the difference between its prescription and Example 1 is only that the propyl gallate in the prescription is replaced with the same amount of butyl ether, and the preparation method is the same as that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com