Application of c-Abl inhibitor in preparation of medicine for preventing and/or treating amyotrophic lateral sclerosis
A lateral sclerosis, c-abl technology, applied in the direction of drug combination, pharmaceutical formula, organic active ingredients, etc., can solve the effect of amyotrophic lateral sclerosis, the effect is not satisfactory, and the amyotrophic lateral sclerosis cannot be completely cured. Accordion sclerosis and other problems, to achieve the treatment or improvement of significant, significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
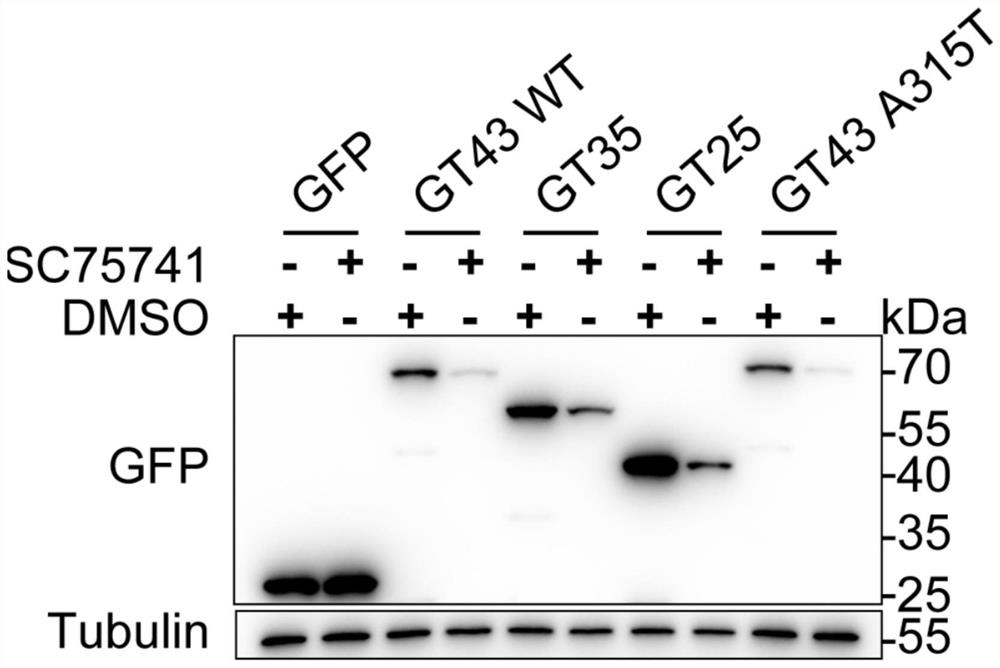
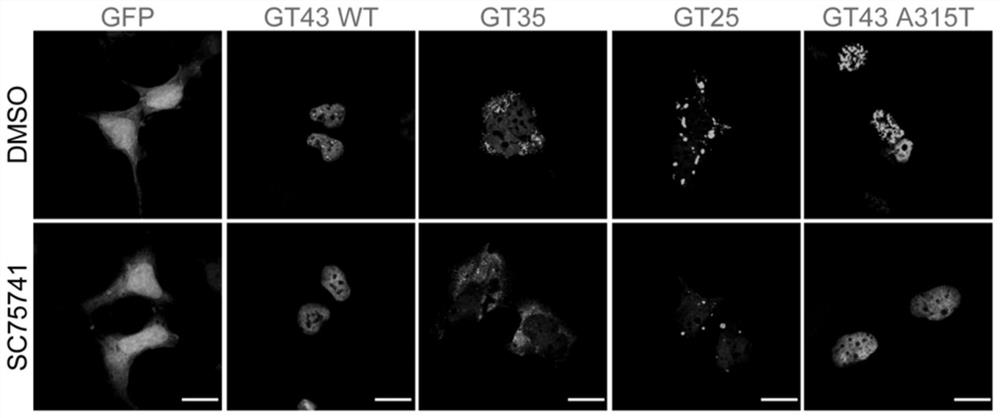
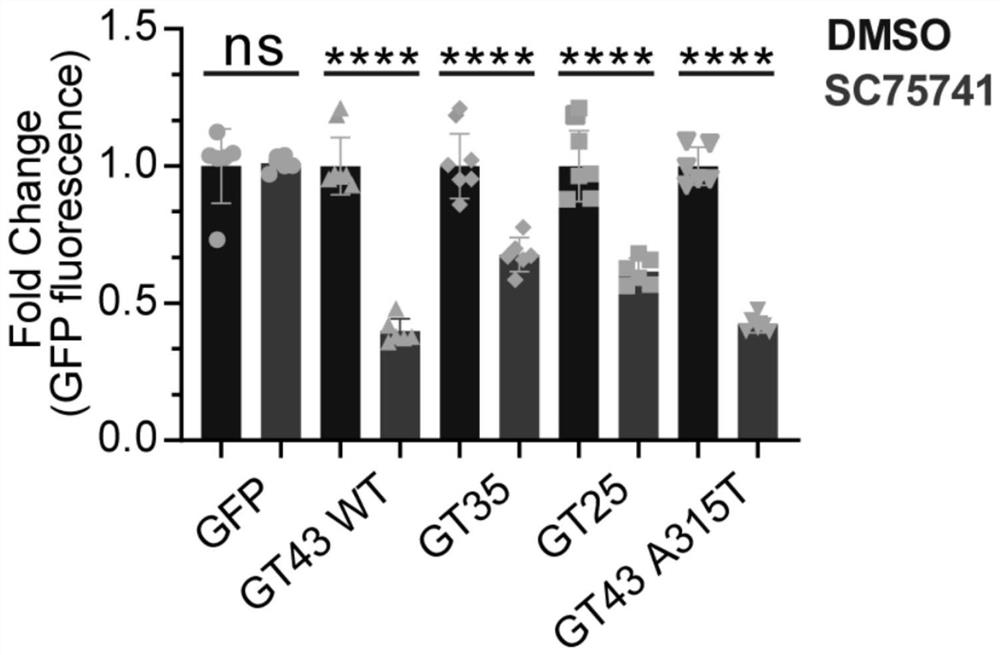
[0105] Example 1. Experiment on degradation of TDP-43 and its related protein aggregates by SC75741
[0106] SH-SY5Y cells in 2*10 5 cells / mL, seeded in 6-well plates at a density of 2 mL per well. After 24 hours, GFP, GT43WT (TDP-43), GT35 (TDP-35), GT25 (TDP-25), GT43 A315T (TDP-43A315T) plasmids were transfected. After 24 hours, cells were treated with SC75741 for 24 hours at a final concentration of 5 μM. Samples were collected with 200 μL of 2XSDS loading buffer. Heating at 100°C for 10 minutes. Immunohybridization: Load 10 μL of each sample, electrophoresis at 100V for 2h; transfer to membrane at 300mA for 1h. 5% skim milk was blocked at room temperature for 1 h, and the antibodies (GFP antibody were purchased from Hangzhou Huaan Biotechnology Co., Ltd., #EM1607-31; Tubulin antibody was purchased from Hangzhou Huaan Biotechnology Co., Ltd., #M1305-2) at a certain dilution ratio (Tubulin antibody was 1:5000, the rest are 1:1000), incubate overnight at 4 degrees, and ...
Embodiment 2
[0112] Example 2. SC75741 degrades amyotrophic lateral sclerosis-related protein aggregate SOD1
[0113] SH-SY5Y cells were seeded in 6-well plates at a density of 2*105 cells / mL, with 2 mL per well. After 24 hours, GFP, GFP-SOD1 G93A (SOD1 G93A mutation produces aggregates) plasmid was transfected. After 24 hours, cells were treated with SC75741 for 24 hours at a final concentration of 5 μM. Samples were collected with 200 μL of 2XSDS loading buffer. Heating at 100°C for 10 minutes. Immunohybridization: Load 10 μL of each sample, electrophoresis at 100V for 2h; transfer to membrane at 300mA for 1h. 5% skim milk was blocked at room temperature for 1 hour, incubated at a certain dilution ratio (1:5000 for Tubulin antibody, and 1:1000 for the rest) overnight at 4 degrees, washed with PBST (phosphate buffered saline + 0.1% Tween20) 3 times for 10 minutes each time . Secondary antibodies (Goat anti-Mouse IgG (H+L) secondary antibodies were purchased from Thermo Fisher Scienti...
Embodiment 4
[0118] Example 4. SC75741 target NF-κB is not involved in SC75741-induced degradation of TDP-43 and its related protein aggregates
[0119] H4GT25 cells (a cell line constructed in this experiment that express TDP-25 induced by doxycycline (Dox) in H4 cells) were seeded in 6-well plates at a density of 2*105 cells / mL, with 2 mL per well. Add 1 μg / μL Dox for 24 h. Add SC75741 (5μM), NF-κB inhibitor: CAPE (10μM), JSH-23 (10μM) for 24 hours. Samples were collected with 200 μL of 2XSDS loading buffer. Heating at 100°C for 10 minutes. Immunohybridization: Load 10 μL of each sample, electrophoresis at 100V for 2h; transfer to membrane at 300mA for 1h. 5% skim milk was blocked at room temperature for 1 h, antibodies (GFP antibody were purchased from Hangzhou Huaan Biotechnology Co., Ltd., #EM1607-31; phospho-c-Abl (Tyr245) was purchased from Cell Signaling #2861; Tubulin antibody was purchased from Hangzhou Huaan Biotechnology Co., Ltd., #M1305-2) at a certain dilution ratio (1:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com