Preparation method of iohexol hydrolysate
A technology of iohexol hydrolyzate and iodide, which is applied in the preparation of organic compounds, carboxylic acid amide preparation, chemical instruments and methods, etc., can solve the problems of high preparation cost, high equipment requirements, and many iodides, and achieve the goal of reaction Fewer by-products, low reaction temperature, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
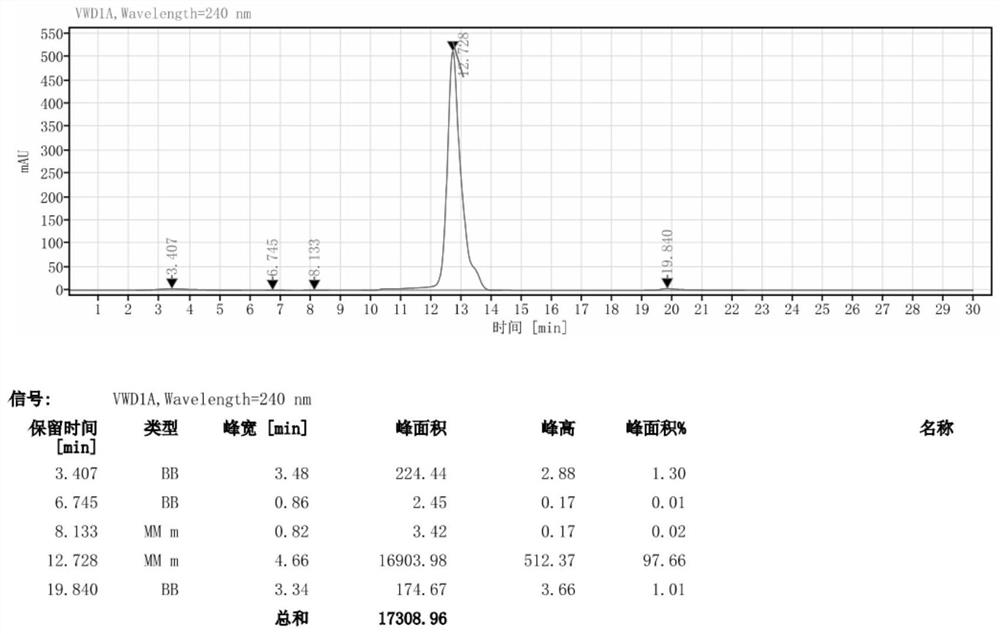
[0030] Add 46g DMAC (N,N-dimethylacetamide, the same below) into a 500ml reaction flask, stir, add 35.3g (0.05mol) iodide, cool down to 5°C, slowly add 35.3g (0.45mol) acetyl chloride dropwise, The dropwise addition was completed in 30 minutes, and the temperature during the dropwise addition did not exceed 20 °C. After the addition was completed, the mixture was stirred for 30 minutes, heated to 50 °C, and kept stirring for 4 hours. HPLC detection showed that the residual raw material was less than 2%; then 100 ml of methanol and 100 ml of water were added. Incubate the reaction at 70°C for 12h, then concentrate under reduced pressure to remove methanol, cool to 5°C, keep stirring for 8h, suction filter, wash the filter cake with water, and blow dry at 60°C for more than 12h to obtain off-white solid iohexol hydrolyzate (II) 15.1 g, the yield is 40.3%, and the purity is 97.66%.
[0031] The purity detection method is the same as the central control method: liquid chromatograp...
Embodiment 2
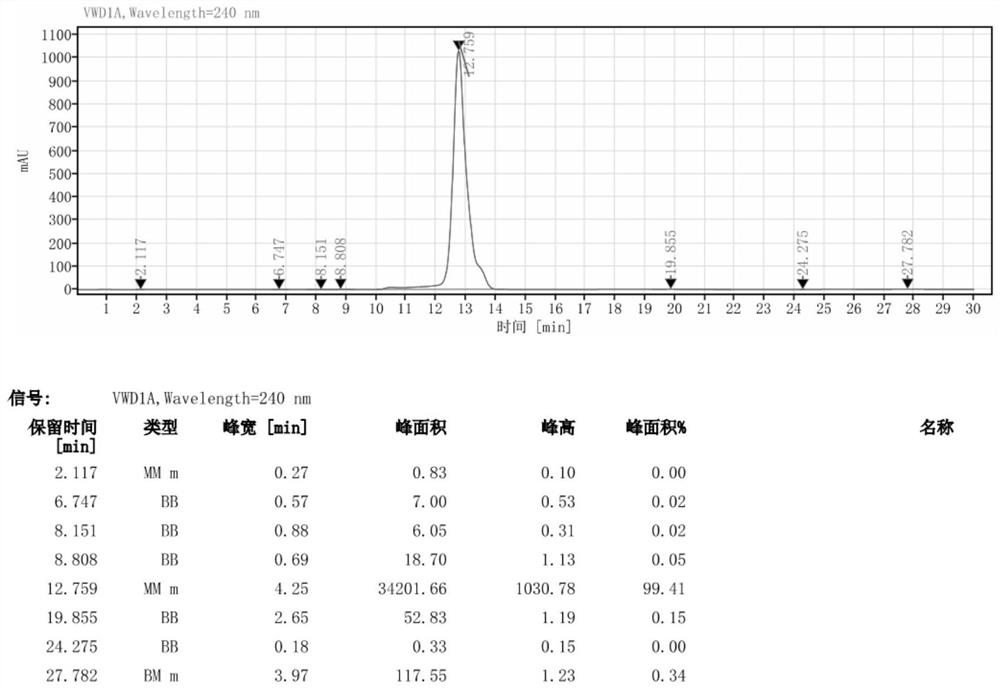
[0034] Add 46g of DMAC to a 500ml reaction flask, stir, add 35.3g (0.05mol) of iodide, cool down to 5°C, slowly add 35.3g (0.45mol) of acetyl chloride dropwise, complete the dropwise addition in 30 minutes, and the temperature during the dropwise addition does not exceed 20 ℃, the addition was completed, stirred for 30 minutes, heated to 50 ℃, kept stirring for 6 hours, detected by HPLC, the reaction of the raw materials was complete, then added 100 ml methanol, kept the reaction at 70 ℃ for 12 hours, cooled to 5 ℃, kept stirring for 8 hours, suction filtration, filter cake After washing with a little water, air drying at 60°C for more than 12 hours to obtain 31.5 g of off-white solid iohexol hydrolyzate (II) with a yield of 84.2% and a purity of 99.41%. The chromatogram of the iohexol hydrolyzate obtained in this example turu figure 2 shown.
Embodiment 3
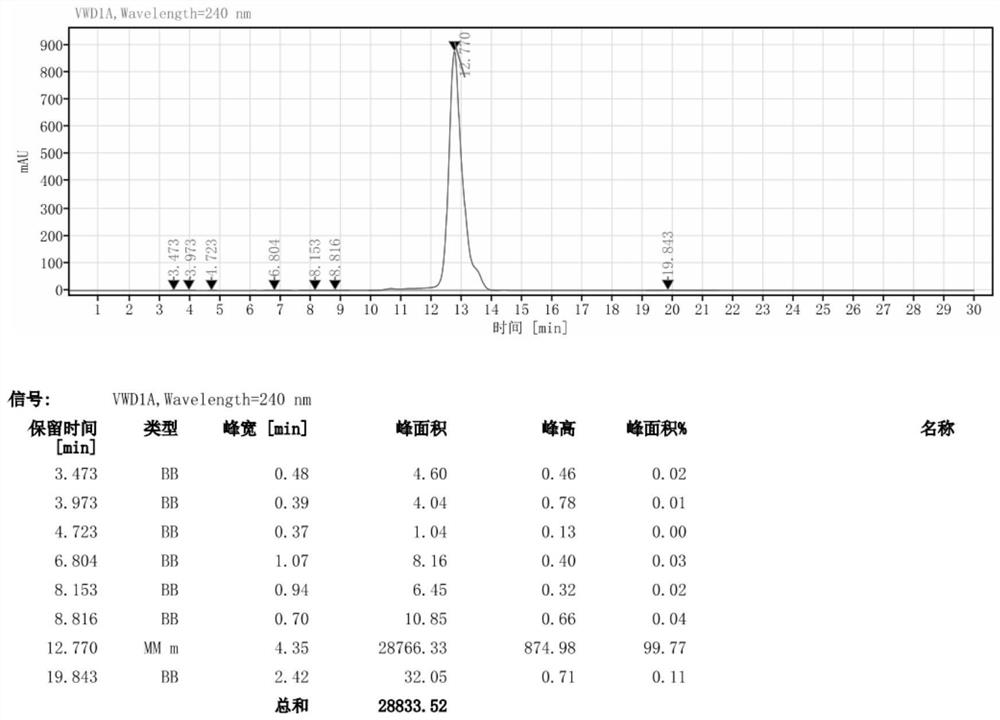
[0036] 1) Add 46g of organic solvent (DMAC) to a 500ml reaction flask, stir, add 35.3g (0.05mol) of iodide, cool down to 5°C, slowly add 35.3g (0.45mol) of acetyl chloride dropwise, complete the dropwise addition in 30 minutes, dropwise During the addition, the temperature did not exceed 20 °C. After the addition was completed, the mixture was stirred for 30 minutes, heated to 55 °C, kept stirring for 6 h, and detected by HPLC. There was almost no raw material remaining; in the specific implementation, the molar ratio of acetyl chloride and iodide (I) was controlled. Within the range of 5.5-10:1, the purpose of the invention can be achieved.
[0037] 2) Cool down to 5°C, then dropwise add 235g of 16% (0.94mol) sodium hydroxide solution, after adding, pH13.4, 45°C for 2 hours;
[0038] 3) Cool down to 5°C, adjust the pH of the solution to 5.8 with 37% acid (hydrochloric acid is used in this example, and in the specific implementation, at least one of hydrochloric acid, sulfuric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com