Method for synthesizing oxazole ring derivative by catalyzing beta, gamma-unsaturated oxime
A synthesis method and derivative technology, applied in the field of organic compound synthesis, can solve the problems of insufficient environmental protection and high cost, and achieve the effect of practical universal path, easy raw materials, and raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Catalytic synthesis of β,γ-unsaturated oxime to 3-(3-methoxyphenyl)-5-methyl-4,5-dihydroisoxazole
[0041] The synthetic reaction formula is as follows:
[0042]
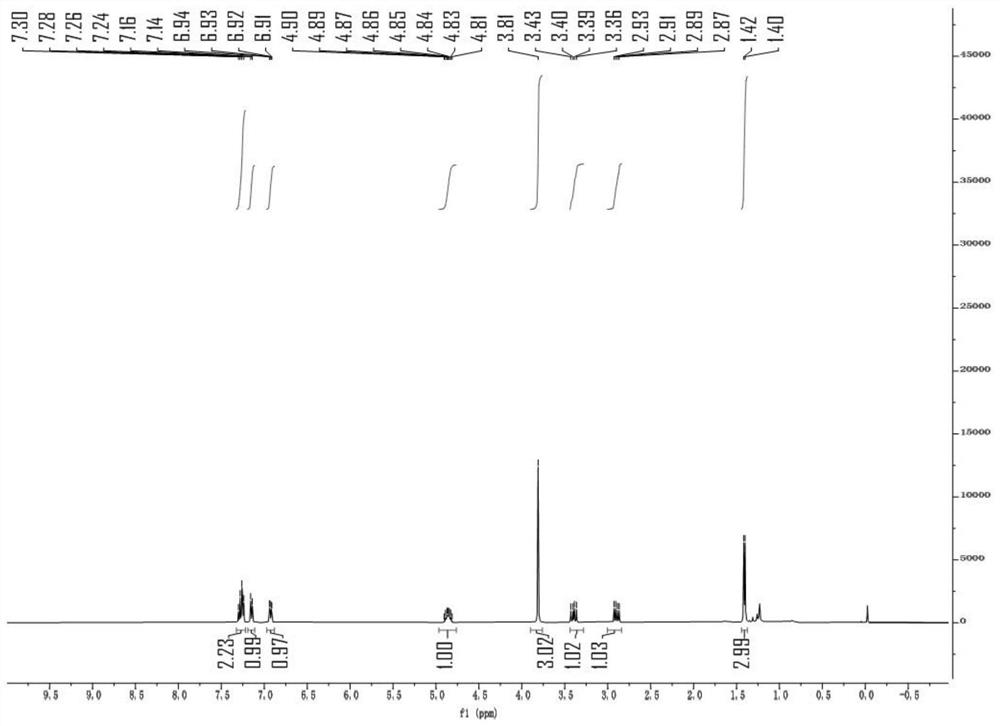
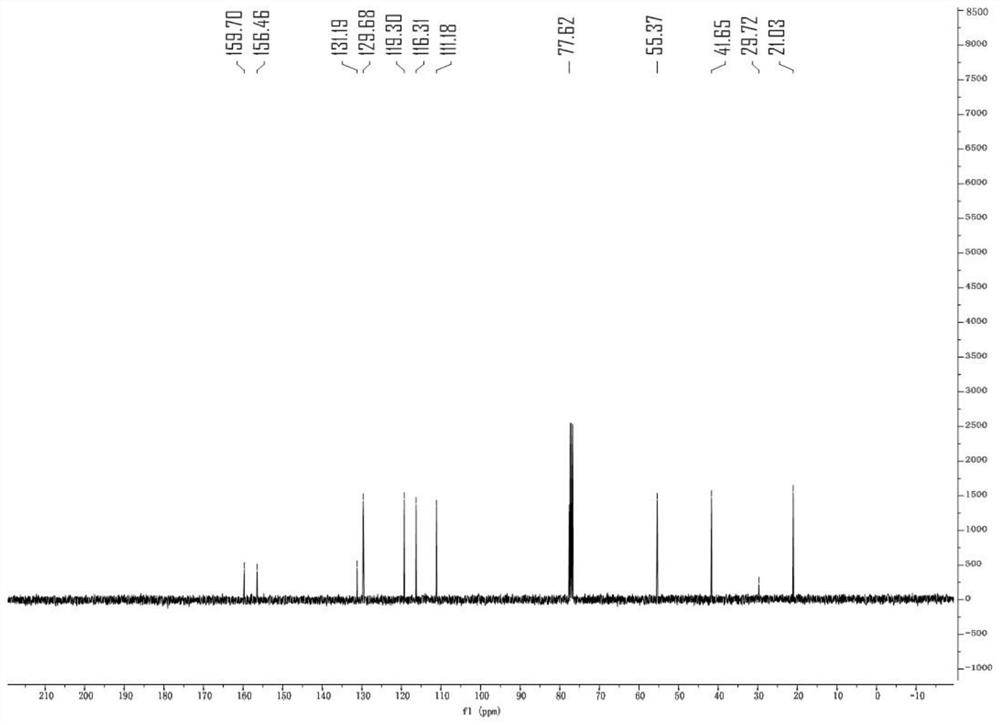
[0043] The specific synthesis method is as follows: 1-(3-methoxyphenyl)but-3-en-1-one oxime (compound 1, 38.2mg) and decacarbonyldimanganese (Mn 2 CO 10 , 7.8 mg) was placed in a 15 mL reaction tube, and after nitrogen was replaced three times, 2 mL of isopropanol (tBuOOH) was added as a solvent under nitrogen flow, followed by tert-butyl hydroperoxide (iPrOH, 36 mg), and the bottle was stoppered. The reaction temperature was raised to 100 °C, the solvent was removed after 4 hours of reaction, and the product compound (I) was obtained by column chromatography: 3-(3-methoxyphenyl)-5-methyl-4,5-di Hydroisoxazole (31 mg, 81% yield). The product is a white solid, NMR data ( figure 1 , 2 )for: 1 HNMR (400MHz, CDCl 3 )δ7.27(q,J=9.1,8.5Hz, 1 H),7.15(d,J=7.6Hz, 1 H),6.93(dd,J=8.3,2.6Hz, 1 H),4....
Embodiment 2
[0044] Example 2 Catalysis of β,γ-unsaturated oxime to synthesize 5-methyl-3-(4-trifluoromethyl)-4,5-dihydroisoxazole
[0045] The synthetic reaction formula is as follows:
[0046]
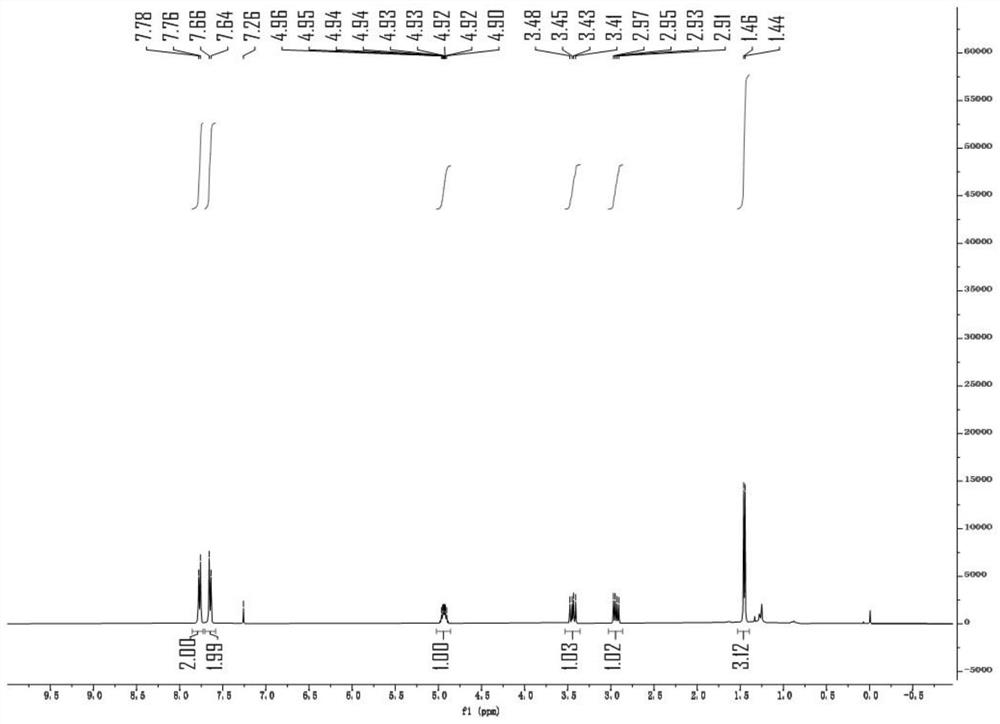
[0047] The specific synthesis method is as follows: 1-(4-trifluoromethyl)but-3-en-1-one oxime (compound 2, 45.8mg) and decacarbonyldimanganese (Mn 2 CO 10 , 7.8 mg) was placed in a 15 mL reaction tube, and after nitrogen was replaced three times, 2 mL of isopropanol (tBuOOH) was added as a solvent under nitrogen flow, followed by tert-butyl hydroperoxide (iPrOH, 36 mg), and the bottle was stoppered. The reaction temperature was raised to 100°C, the reaction was cooled to room temperature after 9 hours, the solvent was removed, and the product compound (II) was obtained by column chromatography: 5-methyl-3-(4-trifluoromethyl) -4,5-Dihydroisoxazole (34.3 mg, 75%), NMR data of the product ( image 3 , 4 )for: 1 H NMR (400MHz, CDCl 3 )δ7.77(d, J=8.1Hz, 1 H),7.65(d,J=8.3Hz, 1 H), 5.00–4.86 ...
Embodiment 3
[0048] Example 3 Catalysis of β,γ-unsaturated oxime to synthesize 3-(p-tolyl)-4,5-dihydroisoxazol-5-yl)-methanol
[0049] The synthetic reaction formula is as follows:
[0050]
[0051] The specific synthesis method is as follows: 1-(p-tolyl)but-3-en-1-one oxime (compound 3,35mg) and decacarbonyldimanganese (Mn 2 CO 10 , 7.8mg) was placed in a 10mL reaction tube, 2mL of trifluoroethanol (TFEA) was added as a solvent, and air was used as an oxidant at 25°C for 1.2 hours of reaction, and then triphenylphosphine (PPh) was added. 3 , 52.4 mg) reacted for 20 min, the solvent was removed and separated by column chromatography to obtain the product compound (III): 3-(p-tolyl)-4,5-dihydroisoxazol-5-yl)-methanol (28.7 mg , the yield is 82%). The product is a colorless liquid, NMR data ( Figure 5 , 6 )for: 1 H NMR (400MHz, CDCl 3 )δ7.54(d, J=7.9Hz, 1 H),7.30–7.13(m, 1 H),4.84(s, 0 H),3.85(dd,J=12.2,3.3Hz, 1 H),3.69(d,J=4.8Hz, 0 H),3.30(dd,J=24.5,9.2Hz, 1 H), 2.37(s, 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com