Intracellular polymerization method for hypoxia response type alkyne-amine click polymerization and application of intracellular polymerization method
A technology of click polymerization, polymerization method, applied in the field of biochemistry, can solve the problem of difficult to distinguish between normal cells and tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
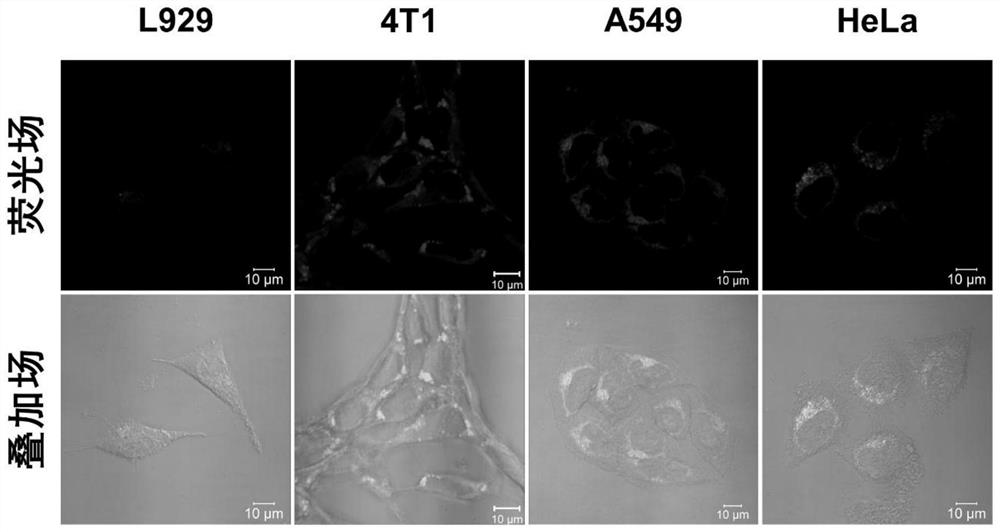
[0071] HeLa cells grown to 80% cell density were digested, resuspended in fresh DMEM (10% fetal bovine serum) medium, and diluted to 1.5 x 10 5 cells / mL, inoculate 1 mL in each laser confocal culture dish, and culture until the cells adhere to the wall, add 150 μM aromatic binary nitro compound 1 solution (solvent is DMEM medium containing 10% fetal bovine serum), Placed at 37°C, 5% O 2 After the hypoxia culture, the culture medium was discarded at room temperature (20 °C), and the cells were carefully washed 3 times with 1 ml of PBS phosphate buffered saline solution, and then the washed cells were added to the concentration of 10 μM bismuth The solution of the alkynyl compound M2 (the solvent is PBS) was placed at 37°C in normoxia (21% O 2 ) cultured for 10 min; after the end of normoxia culture, the culture medium was discarded at room temperature (20°C), and the cells were carefully washed 3 times with 1 ml of PBS phosphate buffered saline solution, and then fresh 10% fet...
Embodiment 2
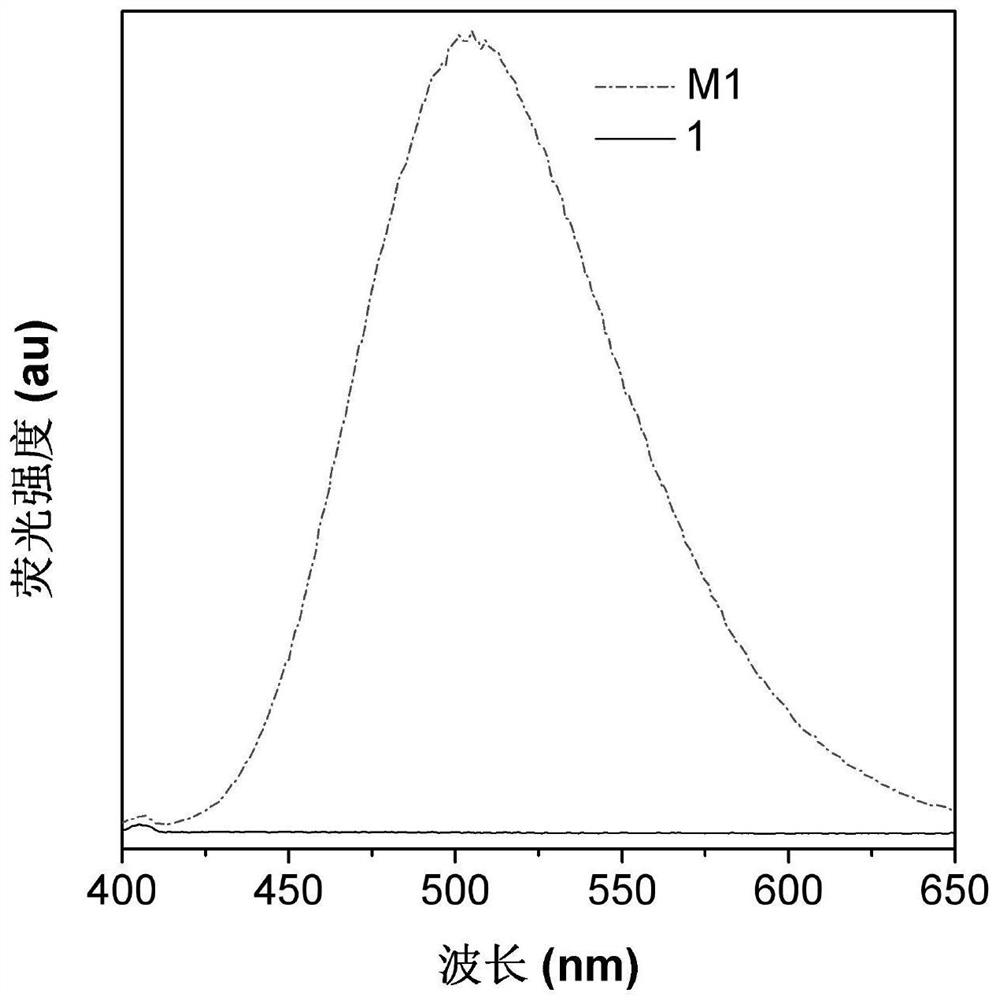
[0079] Detect the luminescence of the aromatic dibasic nitro compound 1 and the aromatic dibasic amine compound M1 in Example 1, and the results are as follows figure 1 shown. from figure 1 It can be seen that compound M1 has strong fluorescence, while compound 1 hardly emits light. It shows that the "lighting" of the fluorescent signal cannot occur before the binary nitro compound is converted into the binary amine compound.
Embodiment 3
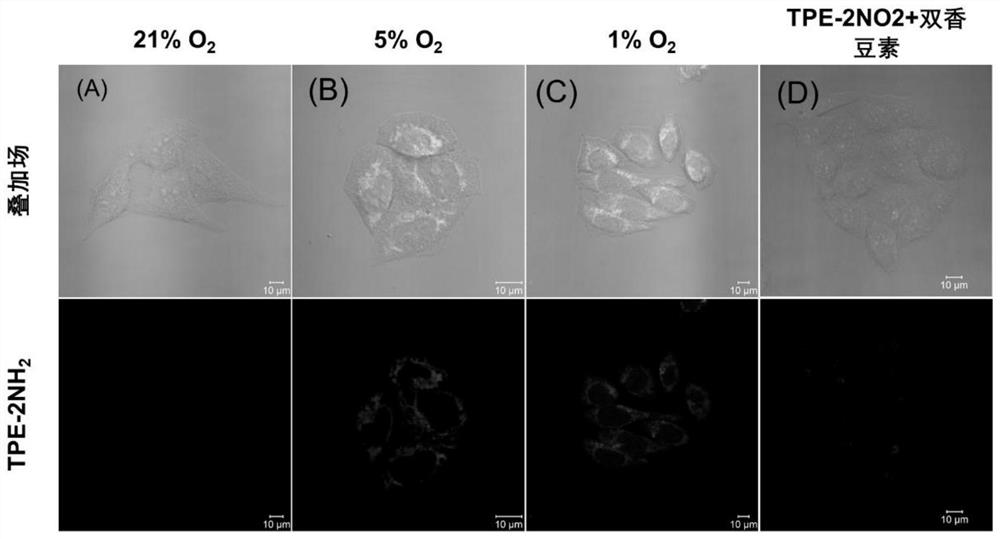
[0081] Set the concentration to 1.5 x 10 5 1 mL of HeLa cells / mL were added to a laser confocal culture dish, and after the cells adhered to the wall, 150 μM of aromatic binary nitro compound 1 solution (the solvent was DMEM containing 10% fetal bovine serum) was added. base) at 37 °C in normoxia (21% O 2 ), 5%O 2 , 1%O 2 and 5% O 2 + Dicoumarin (nitroreductase inhibitor) for incubation, after incubation, the culture medium was discarded at room temperature (20°C), and the cells were carefully washed 3 times with 1 ml of PBS phosphate buffered saline, and then the The washed cells were added with 10 μM dibasic alkynyl compound M2 solution (the solvent was PBS), and then all were cultured in normoxia at 37°C. After the normoxia culture, the culture medium was discarded at room temperature (20°C). , and carefully washed the cells 3 times with 1 ml of PBS phosphate buffered saline solution, and then added fresh DMEM medium containing 10% fetal bovine serum to the washed cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com