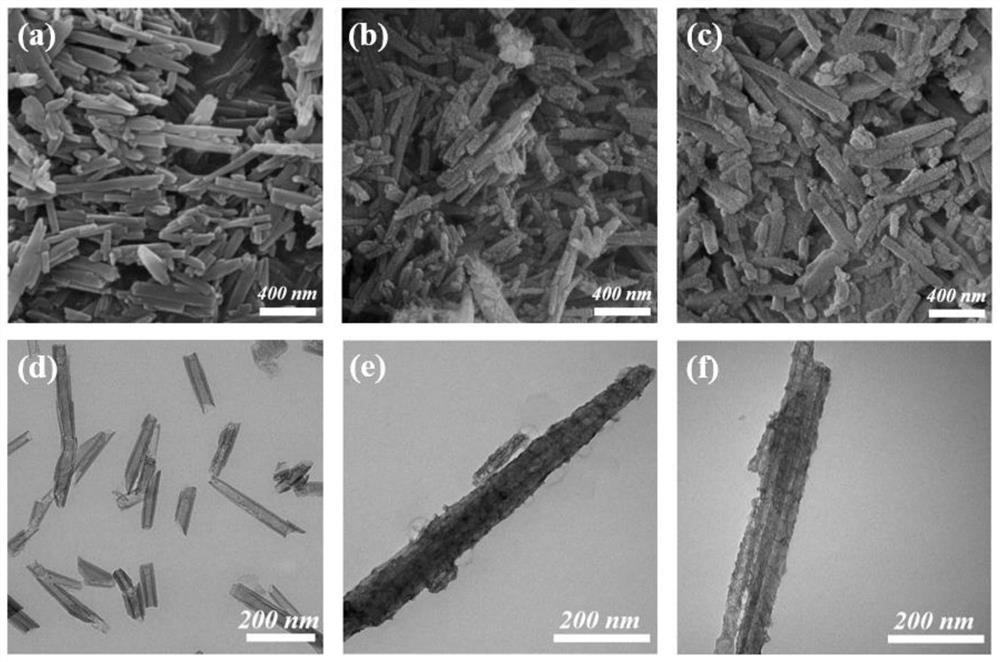
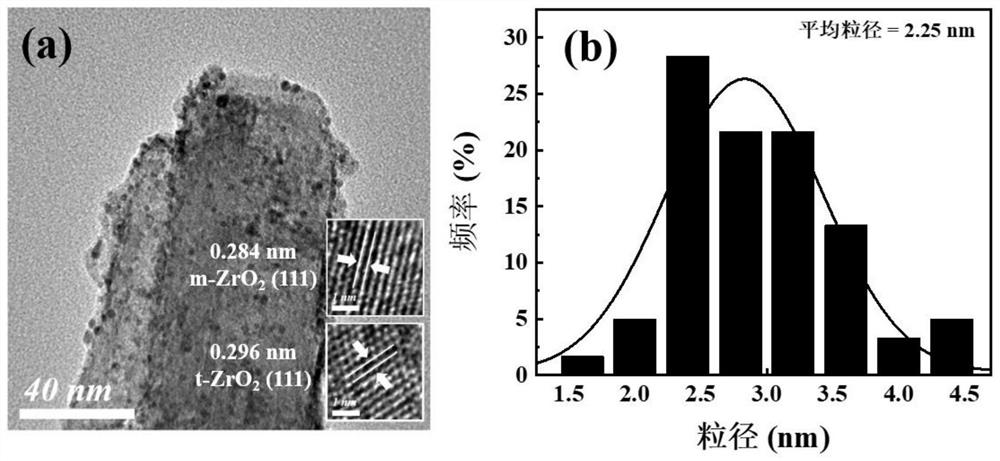
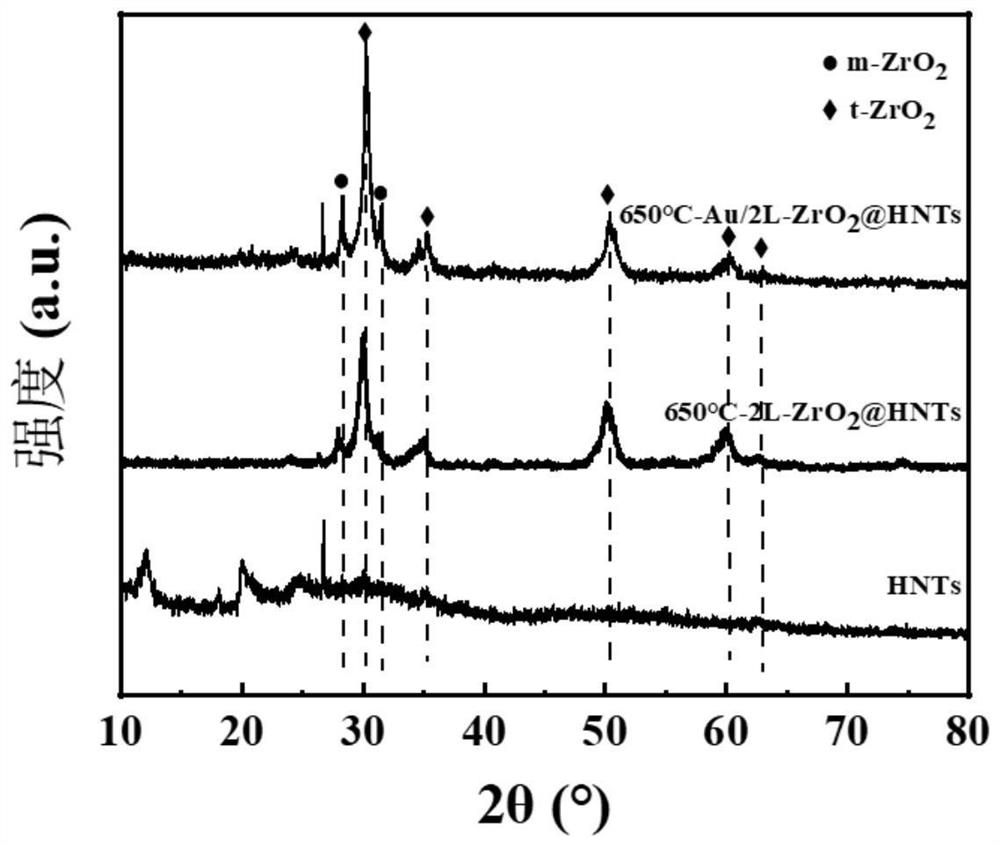
Preparation method and application of oxygen-deficient rod-like core-shell structure catalyst
A core-shell structure and catalyst technology, which is applied in the field of new heterogeneous catalyst preparation, can solve the problems of differences in the number of oxygen vacancies and acid strength, the number of exposed active sites is reduced, and the size of metal nanoparticles is large. ability, improve catalytic activity, and the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Oxygen-deficient rod-like core-shell structure 650℃-Au / 2L-ZrO 2 Preparation of @HNTs catalysts.
[0039] (1) Weigh 40g of HNTs into a three-necked flask, measure 250mL of HNO with a graduated cylinder 3 The solution was placed in a three-necked flask. Then it was placed in an oil bath and a reflux condensing device was installed, and stirred at 75° C. for 12 h. After the reaction was completed, the obtained reaction mixture was washed with deionized water until neutral, and then collected by centrifugation. The obtained samples were dried in a vacuum drying oven at 60 °C for 12 h. The obtained solid was then ground into powder, placed in a tube furnace under an air atmosphere, and calcined at 200° C. for 2 h.
[0040] (2) Weigh 0.23 g of pretreated HNTs and 0.01 g of HPC, disperse them in 20 mL of ethanol, dropwise add 0.1 mL of deionized water, and disperse by ultrasonic to make the samples evenly mixed. The flask was then placed in a 25°C water bath and stirre...
Embodiment 2
[0055] 1. Oxygen-deficient rod-like core-shell structure 450℃-Au / 2L-ZrO 2 Preparation of @HNTs catalysts.
[0056] (1) Weigh 10g of HNTs and place it in a three-necked flask, measure 63mL of HNTs with a graduated cylinder 2 SO 4 The solution was placed in a three-necked flask. Then it was placed in an oil bath and a reflux condensing device was installed, and the mixture was stirred at 75° C. for 8 h. After the reaction was completed, the obtained reaction mixture was washed with deionized water until neutral, and then collected by centrifugation. The obtained samples were dried in a vacuum drying oven at 60 °C for 24 h. The obtained solid was then ground into powder, placed in a tube furnace under air atmosphere, and calcined at 300° C. for 1 h.
[0057] (2) Weigh 0.92 g of pretreated HNTs and 0.04 g of HEC, disperse them in 80 mL of ethanol, dropwise add 0.4 mL of deionized water, and disperse by ultrasonic to make the samples evenly mixed. The flask was then placed in...
Embodiment 3
[0065] 1. Oxygen-deficient rod-like core-shell structure 350℃-Au / 2L-ZrO 2 Preparation of @HNTs catalysts.
[0066] (1) Weigh 20g of HNTs into a three-necked flask, measure 126mL of HNO with a graduated cylinder 3 The solution was placed in a three-necked flask. Then it was placed in an oil bath and a reflux condensing device was installed, and stirred at 80° C. for 8 h. After the reaction was completed, the obtained reaction mixture was washed with deionized water until neutral, and then collected by centrifugation. The obtained samples were dried in a vacuum drying oven at 50 °C for 24 h. The obtained solid was then ground into powder, placed in a tube furnace under air atmosphere, and calcined at 200° C. for 2 h.
[0067](2) Weigh 0.69 g of pretreated HNTs and 0.03 g of HPC, disperse them in 60 mL of ethanol, add 0.3 mL of deionized water dropwise, and disperse by ultrasonic to make the samples evenly mixed. The flask was then placed in a 25°C water bath and stirred for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com