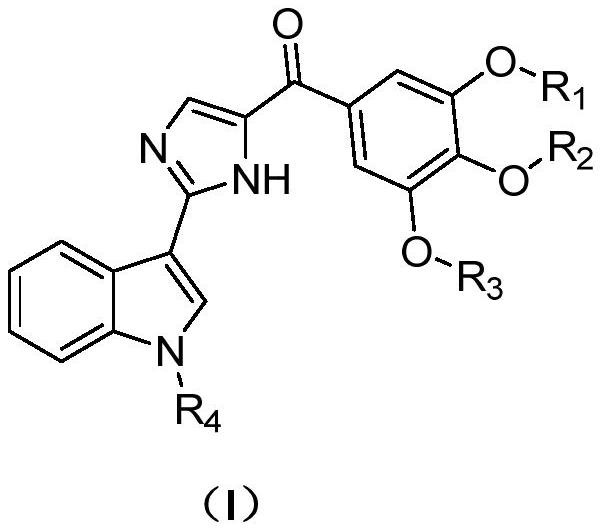
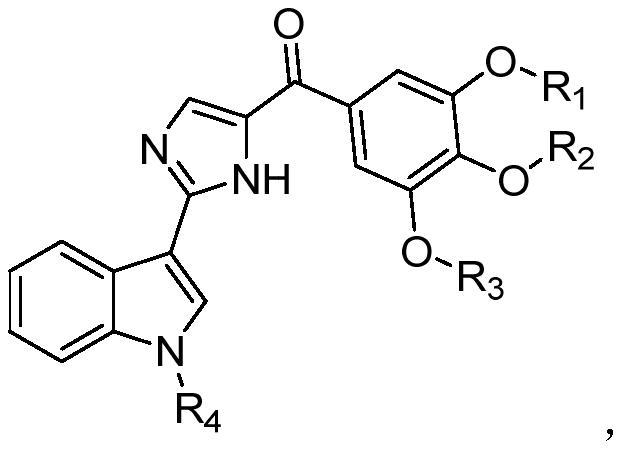
Indole compound for preparing coronavirus therapeutic drug
A coronavirus and compound technology, applied in the field of chemical medicine, can solve the problems of low infectivity, damage to cell export, etc., and achieve the effects of treating viral infection, expanding the time interval of administration, and strong inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: (2-(1H-Indol-3-yl)-1H-imidazol-4-yl)(3,4-dimethoxy-5-(methoxy-d3)phenyl)methanone (Compound 1)
[0032]
[0033] Preparation of 1-(phenylsulfonyl)-1H-indole-5-carbaldehyde (1-2):
[0034]Indole-3-carbaldehyde (1-1) (5 g, 34.5 mmol) was dissolved in ethanol (100 mL) at room temperature, potassium hydroxide (2.1 g, 37.9 mmol) was added and stirred until clear. Concentrate under reduced pressure to remove ethanol, dissolve the residue in acetone (100 mL), and add benzenesulfonyl chloride (6.7 g, 37.9 mmol). Stir at room temperature for 0.5 hour. After filtration, the filtrate was concentrated to obtain crude product, which was recrystallized from methanol to obtain 1-(phenylsulfonyl)-IH-indole-5-carbaldehyde (1-2) (4 g, white solid, yield: 41%). MS-ESI(m / z): 286[M+l] + .
[0035] Preparation of 3-(1 H-imidazol-2-yl)-1-(phenylsulfonyl)-1 H-indole (1-3):
[0036] At 0 °C, 1-(phenylsulfonyl)-1H-indole-3-carbaldehyde (1-2) (4 g, 14.0 mmol) was dissolved in ...
Embodiment 6
[0048] Example 6: (3,4-Dimethoxy-5-(methoxy-d3)phenyl)(2-(1-methyl-1H-indol-3-yl)-1H-imidazole-5 Preparation of -yl)methanone (Compound 6)
[0049]
[0050] The synthetic method refers to Example 1 to obtain (3,4-dimethoxy-5-(methoxy-d3)phenyl)(2-(1-methyl-1H-indol-3-yl)-1H -imidazol-5-yl)methanone (6) (15 mg, yellow solid). MS-ESI(m / z): 395[M+l] + .
[0051] 1 H NMR (400MHz, CD 3 OD)δ: 8.20(s, 1H), 7.86(s, 1H), 7.81(s, 1H), 7.50-7.42(m, 3H), 7.26-7.21(m, 2H), 4.01(s, 3H), 3.96(s, 3H), 3.92(s, 3H).
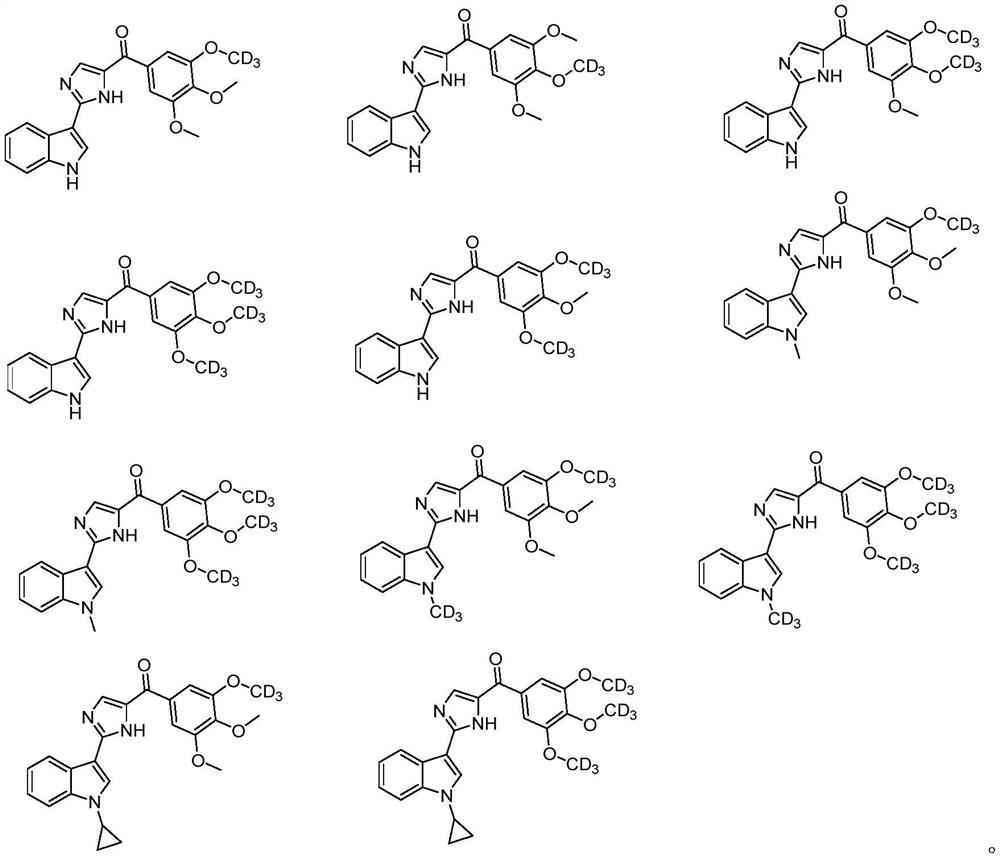
[0052] Examples 7-11 (see Table 2) were synthesized following a basic procedure similar to that of Example 6 to give the desired products.
[0053] Table 2 Structure and data of Examples 7-11
[0054]
[0055]
Embodiment 12
[0056] Example 12: In vitro tubulin polymerization assay
[0057] Bovine brain tubulin (0.4 mg, >97% purity) was mixed with 10 μM of test compound and incubated in 100 μL of the solution in normal tubulin buffer pH 6.9 (80 mM PIPES, 2.0 mM MgCl 2 , 0.5 mM EGTA and 1 mM GTP). Then cool to 0°C, then add GTP, transfer the above mixture to a cuvette, measure the OD value with a spectrophotometer, raise the temperature to 30°C, measure the change in the OD value, thereby reacting the test sample to the microscopic Effects of tubulin assembly. IC 50 For 20 minutes incubation, the concentration of the test sample at which the polymer solubility is inhibited by 50%.
[0058] Table 3 In vitro tubulin inhibitory activity data
[0059] compound Inhibition of tubulin polymerization IC 50 +SD (μM)
[0060] Tubulin assembly experiments were performed on this series of compounds. A total of 3 parallel experiments were carried out, and the experimental results were averaged....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com