High-concentration bacterial ghost vaccine inactivation method
A high-concentration, inactivated technology applied in the biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A19B
[0039] Example 1 A19BG strain inactivation method
[0040] 1. Basic seed propagation
[0041] Take out the constructed strain from the -80°C ultra-low temperature refrigerator, place it in a 37°C constant temperature incubator to thaw, and use a disposable inoculation loop to streak it on a Brucella agar plate (kana plate 100μg / ml) after thawing at 28°C. Cultivate for 5 to 7 days.
[0042] 2 Primary Seed Preparation
[0043] Pick a single colony and put it into 10ml of Brucella broth for 24h at 28°C, 160r / min.
[0044] 3 Secondary Seed Preparation
[0045]Take the prepared first-class seeds and add them to a 500ml conical flask containing 200ml of Brucella broth medium at a 2% inoculation ratio. Take 100mg / mL kanamycin and add it to the culture at a ratio of 1:1000. medium, 28°C, 300r / min, and cultured for 24h.
[0046] 4 Antigen culture
[0047] Put the bacterial liquid in the seed tank into the fermenter, take 100mg / mL kanamycin, add it to the Brucella broth at a ratio...
Embodiment 2
[0057] Example 2 Selection of culture duration and pouring time interval
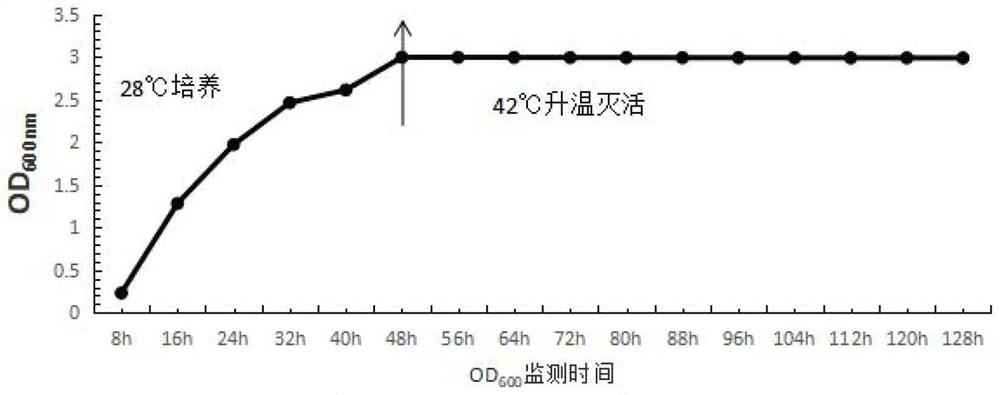
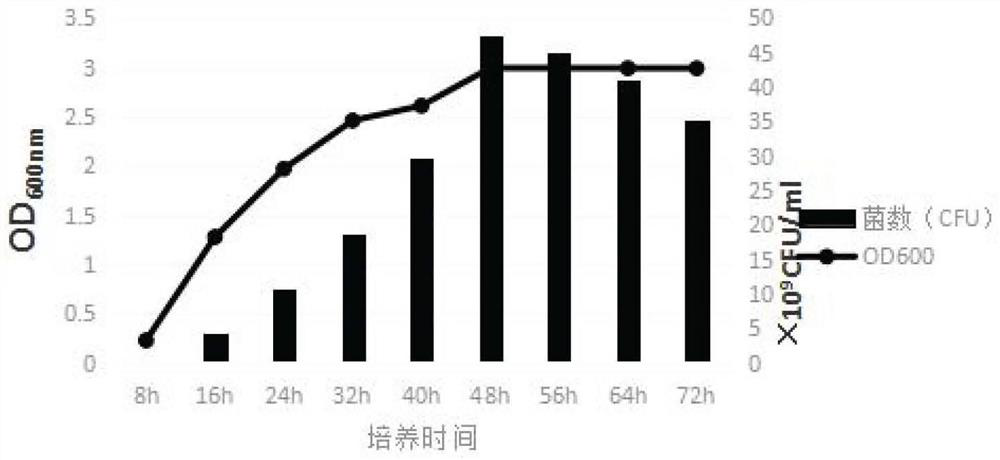
[0058] Adopt the method of multiplying and enlarging culture in Example 1, take samples every 8h to carry out OD 600 Value detection, the growth curve of A19BG strain was measured, and the results are shown in figure 2 .
[0059] The results showed that the bacterial concentration reached 4.7 × 10 after 48 hours of culture. 10 CFU / ml.
[0060] Then the pair concentration is 4.7×10 10 The bacterial liquid of CFU / ml was inactivated and counted. The specific test groups and test results are shown in Table 2.
[0061] Table 2 OD values of different pouring time intervals
[0062]
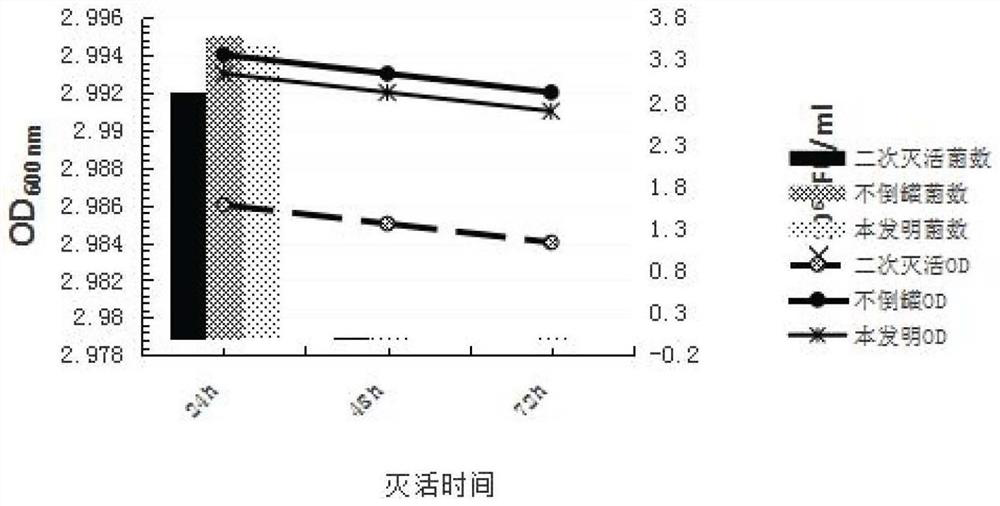
[0063] The results in Table 2 show that the antigen can be completely inactivated when the inactivation interval is 8h, 12h, and 24h within a certain inactivation culture time. On the basis of the time (8 hours), the interval between pouring tanks can be appropriately extended to ensure that the removal effect of bact...
Embodiment 3
[0064] Example 3 Inactivation effect of different bacterial liquid concentrations
[0065] Using the propagation, amplification and inactivation culture methods in Example 1-2, the A19BG strain was cultured with antigen, and the OD was sampled every 8h. 600 value detection, and the concentration of the bacterial solution reaches 5.0 × 10 10 CFU / ml, 5.5×10 10 CFU / ml, 6.0×10 10 When CFU / ml, the bacterial liquid is inactivated and counted to verify the inactivation effect of the present invention. The specific detection results are shown in Table 3.
[0066] Table 3 Inactivation effects of different bacterial concentrations
[0067]
[0068] The results show that the inactivation method of the present invention can kill bacteria with a concentration of 5.0×10 10 CFU / ml, 5.5×10 10 CFU / ml, 6.0×10 10 CFU / ml bacterial solution was completely inactivated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com