Antibodies binding rankl and uses thereof
A monoclonal antibody, antigen technology, applied in the direction of antibodies, antibody medical components, medical preparations containing active ingredients, etc., can solve problems such as infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0126] Preparation of monoclonal antibodies of the present application
[0127] The monoclonal antibodies (mAbs) of the present application can be prepared using the somatic hybridization (hybridoma) technique known in the art of Kohler and Milstein (1975) Nature 256:495. Other embodiments of making monoclonal antibodies include viral or oncogenic transformation of B lymphocytes and phage display techniques. Chimeric or humanized antibodies are well known in the art. See, eg, US Patents 4,816,567; 5,225,539; 5,530,101; 5,585,089; 5,693,762; and 6,180,370, the contents of which are incorporated herein by reference in their entirety.
[0128] Generation of transfectomas producing monoclonal antibodies of the present application
[0129]Antibodies of the present application can also be produced in host cell transfectomas using, for example, a combination of recombinant DNA techniques and gene transfection methods well known in the art (eg, Morrison, S. (1985) Science 229:12...
Embodiment 1
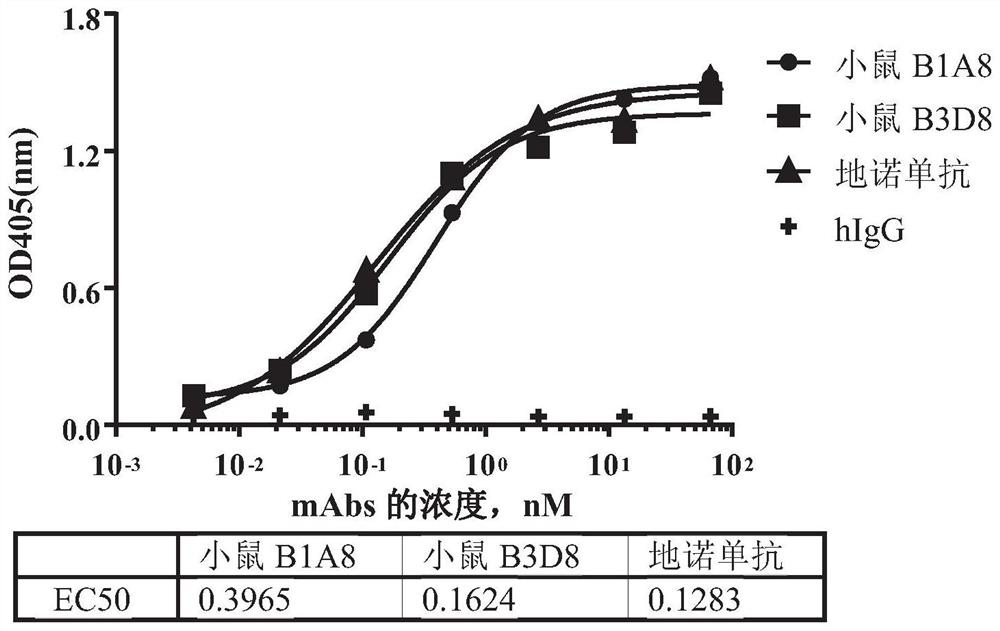
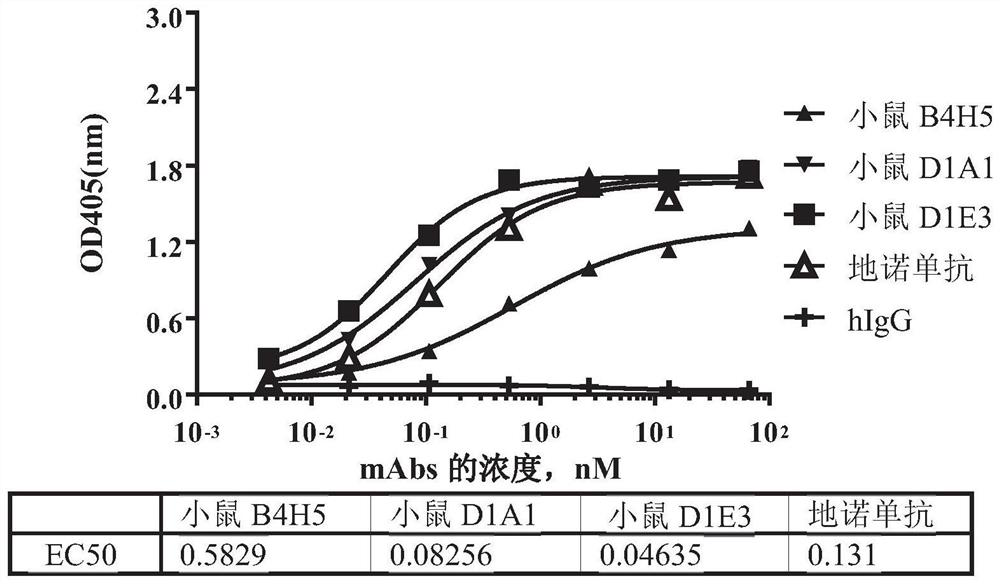
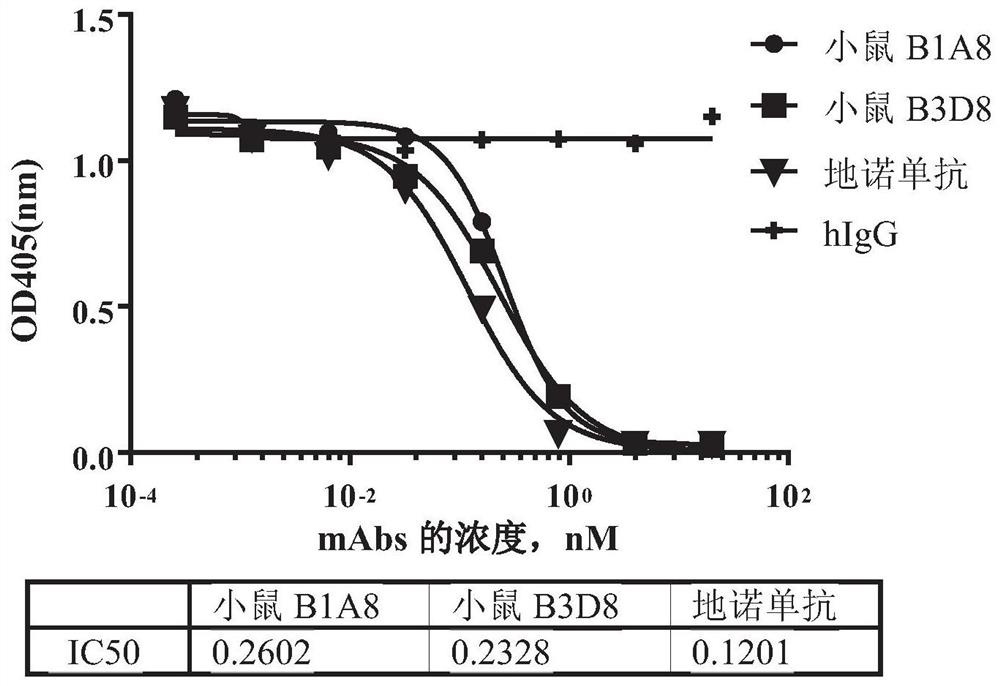
[0166] Example 1. Generation of mouse-derived RANKL monoclonal antibody using hybridoma technology
[0167] immunity
[0168] Mice were immunized according to the method described in E Harlow, D. Lane, Antibody: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 1998. Recombinant human RANKL protein (amino acid sequence 136-317, uniprot #014788) was prepared in-house with human IgG1 Fc at the C-terminus as an immunogen, also for determination of antiserum titers and screening of hybridomas secreting antigen-specific antibodies.
[0169] The immunization dose contained 25 μg human RANKL-Fc protein / mouse / injection for primary and booster immunizations. To increase the immune response, Freund's complete adjuvant and Freund's incomplete adjuvant (Sigma, St. Louis, Mo., USA) were used in primary and booster immunizations, respectively. Briefly, adjuvant-antigen mixtures were prepared as follows. First, mix the adjuvant in the vial using a vorte...
Embodiment 2
[0172] Example 2. Affinity determination of mouse RANKL monoclonal antibody using Octet biofilm interferometry
[0173]The purified RANKL mouse-derived monoclonal antibody produced in Example 1 was characterized for affinity and binding kinetics using the Octet system (ForteBio, Octet RED 96).
[0174] Briefly, AHC / AMC biosensors (anti-human / anti-mouse IgG Fc capture, ForteBio) were pre-soaked in 10 mM glycine (pH 1.5) for 3 sec before immersion in flow buffer containing 0.5% w / v BSA in the wells of PBST) for 3 seconds and the above soaking and immersion steps were repeated three times. After immersing the sensor in HBS-EP containing + 5.0 μg / mL of the RANKL antibody of the present application or the control reference (denosumab, prepared with the heavy and light chains of amino acid sequences of SEQ ID NOs: 38 and 39, respectively) in the wells for 100 seconds, followed by immersion in HBS containing -EP + hole for 5 minutes. in another containing HBS-EP + 180 seconds ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com