Application of NK (Natural Killer) cells, NK cell reinfusion preparation and combined preparation
A technology of NK cells and preparations, applied in the field of biomedicine, can solve the problems of limited prevention and treatment effect of antiviral drugs, cytomegalovirus infection, virus-resistant strains, etc., to promote HCMV clearance, NK cell phenotype maturation, prevention Effects of HCMV infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Mouse model experiment:
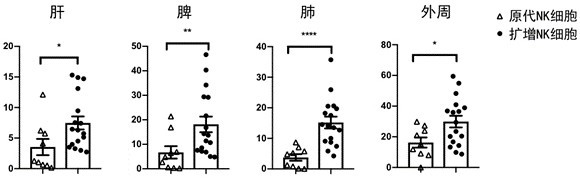
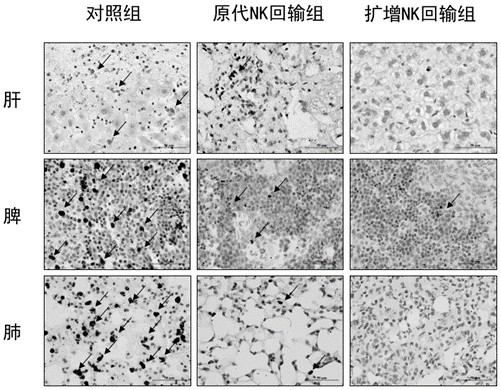
[0061] After 6-8 weeks of female NSG mice were irradiated with a sublethal dose of X-ray, HCMV-IGG seropositive G-CSF-mobilized peripheral blood stem cells were reinfused through the tail vein 1×106 / mouse; 2 weeks later , MRC-5 cells infected with HCMV virus strain AD169 were injected intraperitoneally; 4 weeks after transplantation, 1 × 10 NK cells or primary NK cells were adoptively reinfused. 7 50,000 units of IL-2 were injected intraperitoneally every other day after the NK cells were reinfused. The liver, spleen and lung of mice were harvested on the 14th day after NK cell reinfusion, and flow cytometry was used to detect the proportion of NK cells; based on in situ hybridization, HCMV-RNA probe was used to detect the number of HCMV-positive cells.
[0062] figure 1 The proportion of NK cells in the liver, spleen, lung, and periphery after 14 days of NK cell infusion is shown.
[0063] Depend on figure 1 It can be seen that a...
Embodiment 2
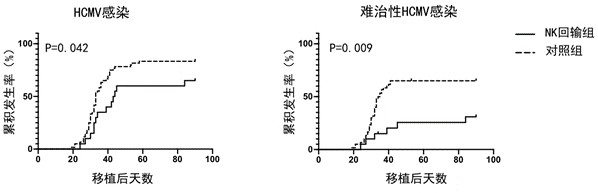
[0066] Example 2 Clinical study:
[0067] 10 days before the donor's haplotype hematopoietic stem cell transplantation, collect 60-80 ml of peripheral blood from the donor (determined by the WBC count in the donor's peripheral blood), or collect peripheral blood stem cells (3-4 ml) after the donor's mobilization, and separate PBMCs Cryopreservation, those who meet the inclusion criteria, the donor PBMC2*10 will be resuscitated 10 days after transplantation 7 , to initiate donor-derived NK culture.
[0068]20 patients who underwent haplotype hematopoietic stem cell transplantation were selected to be included as NK reinfusion objects. Enrollment conditions: (1) Diagnosed as: acute leukemia, or MDS, MM or lymphoma. (2) Aged 16-65 years old; (3) Donor seropositive for HCMV-IGG; (4) Negative for HCMV within 20 days after transplantation; (5) No grade 1-4 aGVHD occurred within 20 days after transplantation, or aGVHD has been resolved and The dose of glucocorticoid < 0.5 mg / kg / d w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com