Preparation method and application of cyclic ether type diaryl heptane in juglans regia
A technology of diarylheptane and Qinglongyi is applied in the field of medicine, which can solve problems such as pollution, and achieve the effects of reducing environmental pollution and improving effective utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 3',4''-Epoxy-1-(4'-methoxyphenyl)-7-(2'',6''-dihydroxy-3''-methoxyphenyl)-3- The preparation method of heptanone:
[0028] (1) Alcohol extraction method: dry fresh Qinglongyi at low temperature, take 10 kg and pulverize it into coarse powder, add 8 times the volume of 95% ethanol, extract by heating under reflux for 3 times, combine the obtained extracts, recover the solvent, and reduce the pressure. After drying, 688.8 g of extract was obtained;
[0029] (2) Organic solvent extraction method: dilute the extract obtained in step (1) by adding 5 times the amount of pure water, and then extract the diluted solution 5 times with the same volume of dichloromethane as the diluent, and combine the dichloromethane. The methane extract was concentrated and dried to obtain 238.6 g of dichloromethane extract;
[0030] (3) Purification and enrichment of Girard reagent: take the dichloromethane extract obtained in step (2), repeatedly knead and dissolve it in 5000 mL of acetic ac...
Embodiment 2
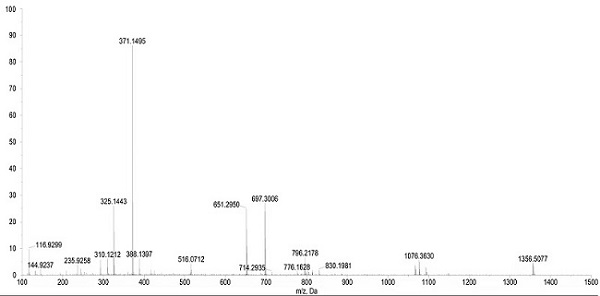
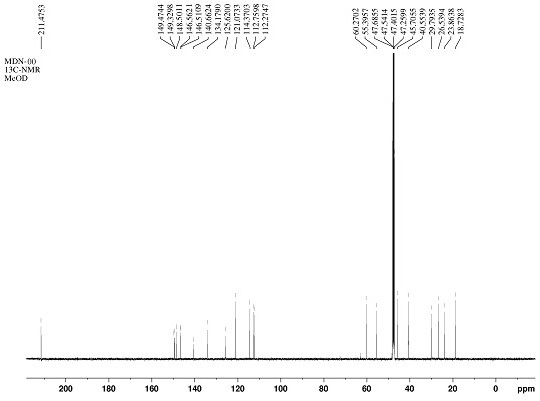
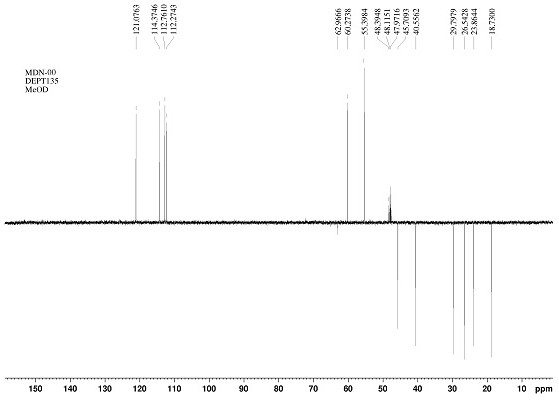
[0034] The mass spectrum and NMR spectrum data of the compound of the present invention are as follows:
[0035] HR-ESI-MS m / z: 371.1495 [M-H] - , indicating that the molecular weight of this compound is 372. 1 H-NMR (CD 3 OD, 600 MHz) δ: 1.48 (2H, m, H-5), 1.63 (1H, m, H-6a), 1.71 (1H, m, H-4a), 1.77 (1H, m, H-6b) , 2.18 (1H, dt, J = 19.4, 8.2 Hz, H-4b), 2.28 (1H, m, H-2a), 2.33 (1H,m, H-7a), 2.35 (1H, m, H-2b), 2.61 (1H, dd, J = 16.0, 8.2 Hz, H-1a), 2.92 (1H,dd, J = 16.0, 10.2 Hz, H-1b), 3.13 (1H, dt, J = 13.0, 5.2 Hz, H-7b), 3.80 (3H,s, H-7'), 3.86 (3H, s, H-7''), 5.50 (1H, d, J = 1.6 Hz, H-2'), 6.03 (1H, s , H-5''), 6.62 (1H, dd, J = 8.0, 1.6 Hz, H-6'), 6.83 (1H, d, J = 8.0 Hz, H-5'); 13 C-NMR (CD 3 OD, 150 MHz) δ: 18.7 (C-5), 23.9 (C-6), 26.5 (C-1), 29.8 (C-7), 40.6 (C-2), 45.7 (C-4), 55.4 (C-7') , 60.3 (C-7''), 112.3 (C-5'), 112.8 (C-2'), 114.4 (C-5''), 121.1 (C-6'), 125.6 (C-1'' ), 134.2 (C-1'), 140.7 (C-3''), 146.5 (C-4'), 146.6 (C-4''), 148.5 (C-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com