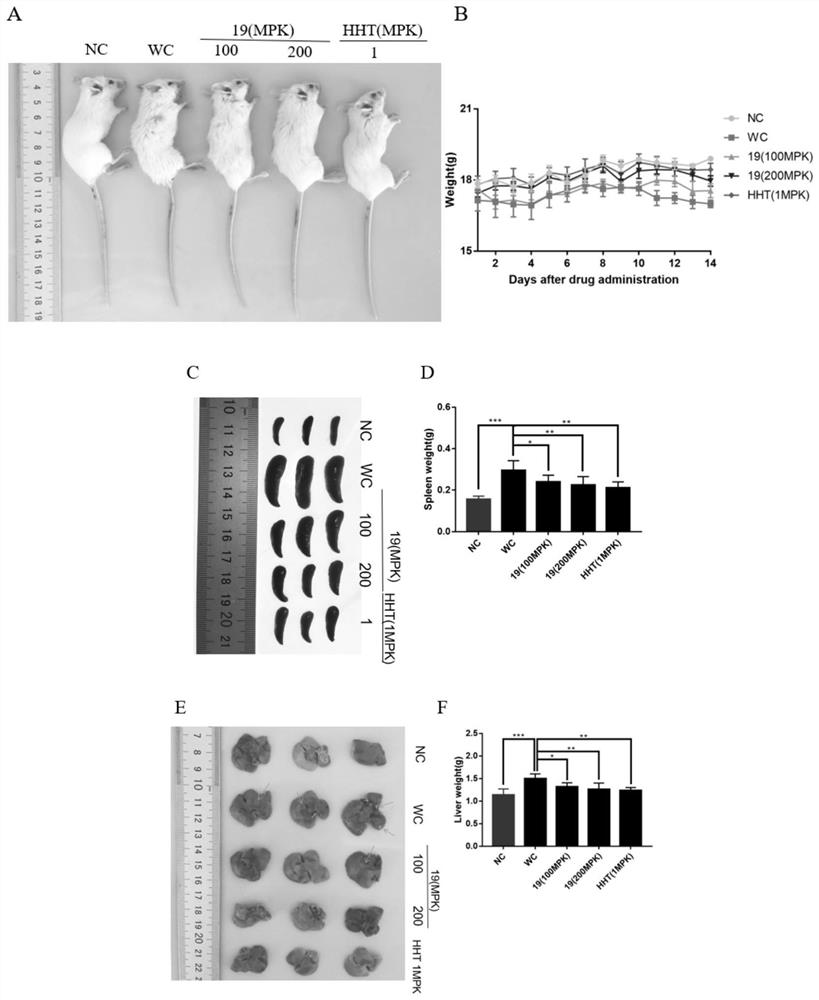
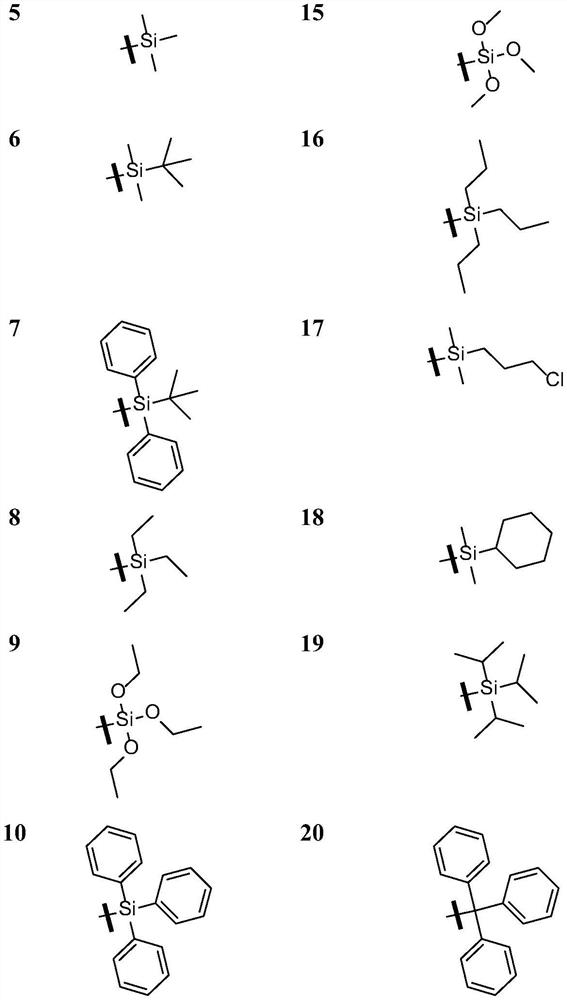
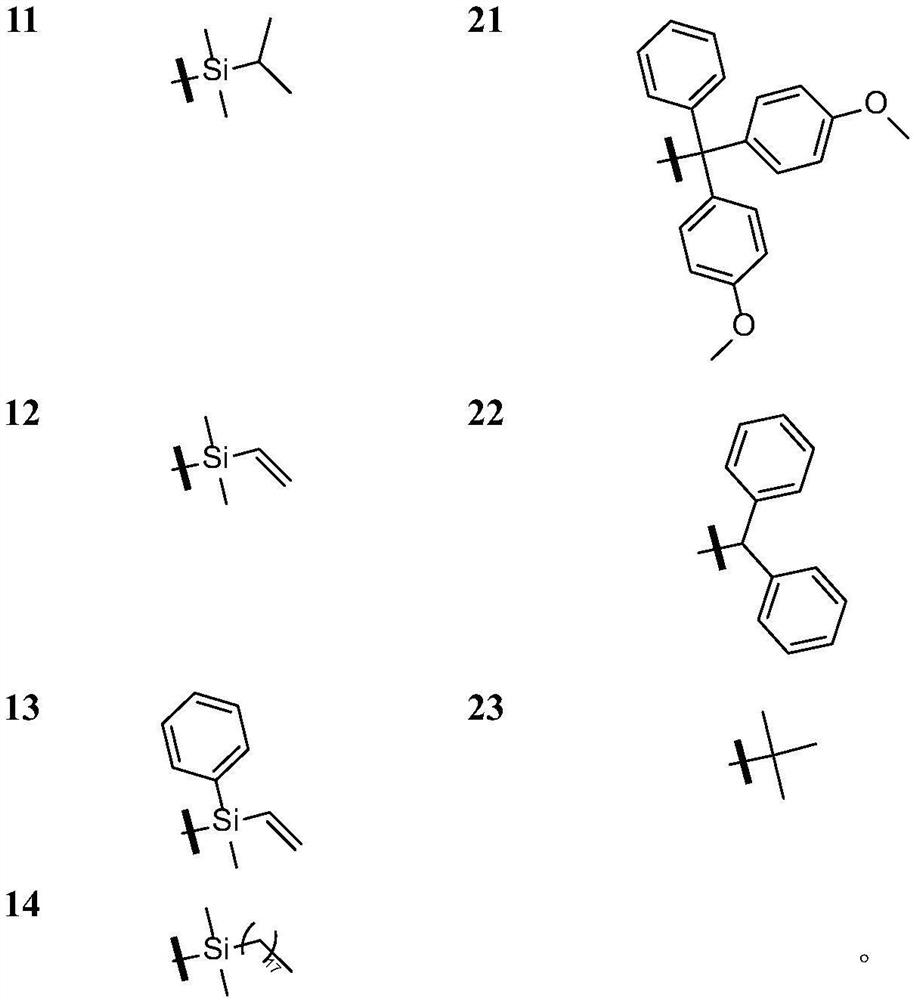
Grain lactone derivative as well as preparation method, pharmaceutical composition and application thereof
A derivative, the technology of glutaractone, which is applied in the field of chemical medicine, can solve the problems of no anti-tumor activity of glutaractone derivatives and insufficient killing ability of cancer cells, and achieve excellent anti-leukemia cell activity, strong tumor cell proliferation, and obvious anti-tumor activity. The effect of tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Preparation of Compound 4
[0020] The structure of compound 4 is as follows:
[0021]
[0022] (1) Preparation of compound 1
[0023] The structure of compound 1 is as follows:
[0024] The preparation process is as follows:
[0025] Trifluoroacetic acid (66.6 mL, 900 mmol) was dissolved in dichloromethane (300 mL), and sodium borohydride (4.54 g, 120 mmol) was slowly added to the reaction system at -10 °C. g, 30.0 mmol) in dichloromethane (10 mL) was slowly added dropwise to the above reaction system, reacted at 0°C for 20 minutes, then slowly added sodium carbonate to neutralize the reaction system, washed with dichloromethane and washed with saturated sodium chloride solution , the organic phase was collected, concentrated and dried, and purified by column chromatography (dichloromethane:methanol=40:1) to obtain compound 1 (white solid, 5.61 g, 83%).
[0026] (2) Preparation of compound 2
[0027] The structure of compound 2 is as follows:
[0...
Embodiment 2
[0034] Example 2: Preparation of Compound 5
[0035] The structure of compound 5 is as follows:
[0036] The preparation process is as follows:
[0037] To a solution of compound 4 (236 mg, 1.00 mmol) in dichloromethane (5 mL) at 0 °C, imidazole (340 mg, 5.00 mmol) and trimethylchlorosilane (327 mg, 3.00 mmol) were added, and the organic phase was collected after filtration for 1 h. Concentrated and dried, and purified by column chromatography (petroleum ether:ethyl acetate=60:1) to obtain compound 5 (white solid, 256 mg, 83%).
[0038] Compound 5 is detected, and its detection results are as follows: 1H NMR(400MHz, Chloroform-d)δ6.42(s,1H),5.54(d,J=2.7Hz,1H),4.62(d,J=3.6Hz,1H),3.59(t,J=7.5Hz , 1H), 2.71–2.47 (m, 2H), 2.28 (d, J=6.5Hz, 1H), 2.05–1.70 (m, 4H), 1.55–1.22 (m, 5H), 0.75 (d, J=5.3 Hz,3H),0.05(s,9H). 13 C NMR (100MHz, CDCl 3 ) δ166.2, 132.2, 128.8, 81.1, 79.9, 43.1, 39.5, 34.2, 31.3, 31.1, 30.1, 27.6, 22.3, 10.5, 0.3.
Embodiment 3
[0039] Example 3: Preparation of Compound 6
[0040] The structure of compound 6 is as follows:
[0041] The preparation process is as follows:
[0042] Using tert-butyldimethylsilyl chloride (453 mg, 3.00 mmol), following the synthetic procedure for compound 5 described in Example 2, the title compound 6 (white solid, 302 mg, 61%) was obtained.
[0043] Compound 6 is detected, and its detection results are as follows: 1 H NMR (400MHz, Chloroform-d) δ6.41(s,1H), 5.53(s,1H), 4.61(s,1H), 3.60(t, J=8.8Hz,1H), 2.79–2.46(m, 2H), 1.98(d, J=15.2Hz, 2H), 1.89-1.73(m, 1H), 1.55(m, 2H), 1.46-1.38(m, 1H), 1.33(m, 2H), 1.26(m ,2H),0.86(s,9H),0.75(s,3H),-0.02(s,6H). 13 C NMR (100MHz, CDCl 3 )δ166.1,132.1,128.6,81.0,79.8,43.3,39.3,34.1,30.1,29.7,27.5,25.8,22.3,18.1,10.5,-4.5,-4.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com