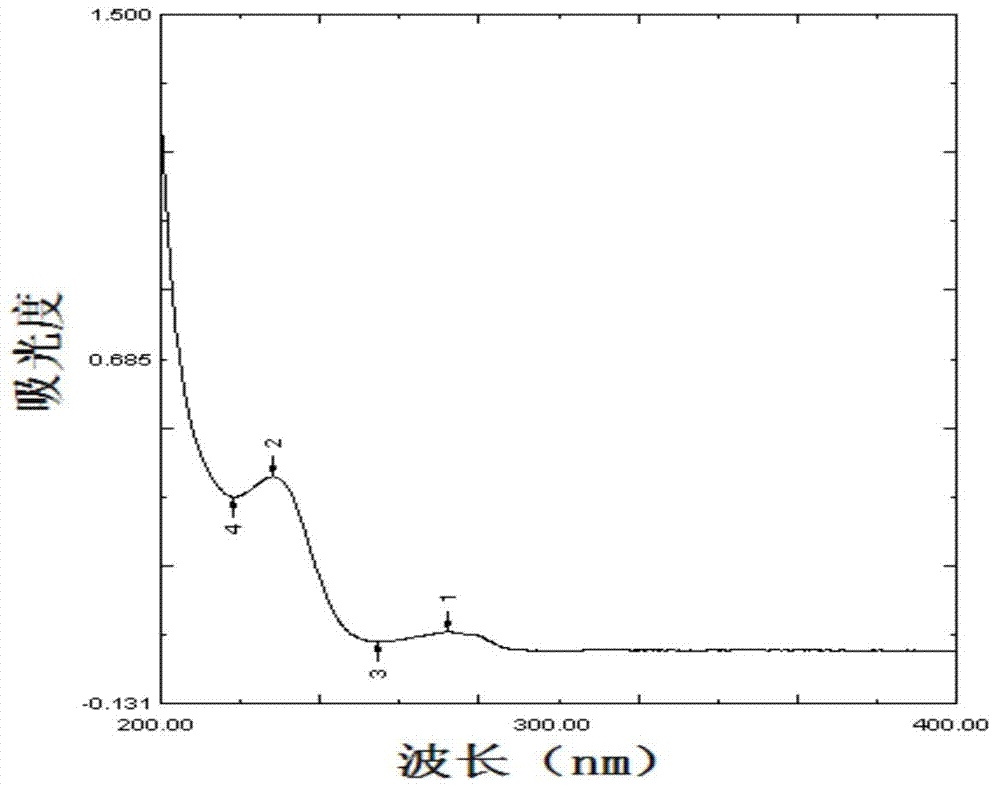
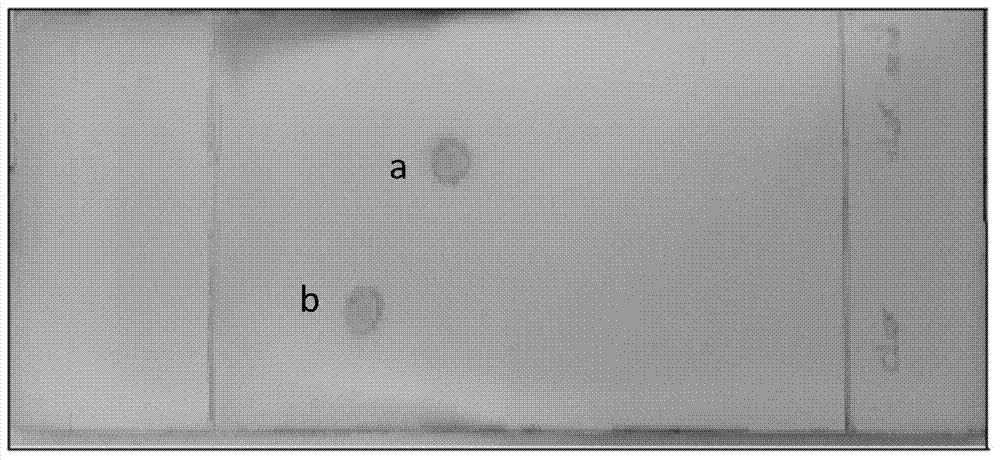
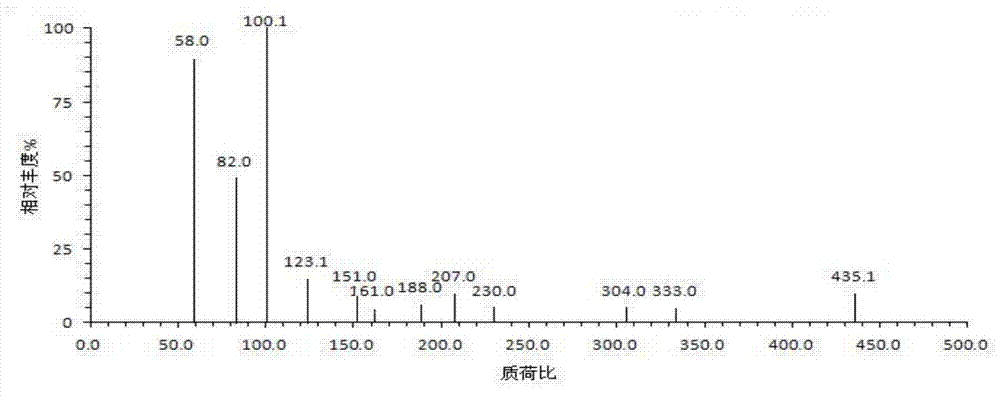
(5S, 6R) cloxacillin penicilloate as well as preparation method and application thereof
A technology of cloxacillin penicillin thiazole salt and cloxacillin, which is applied in the field of drug impurity preparation, can solve problems such as unfavorable application, high impurity content in products, and great influence on finished product quality, and achieves simple and easy preparation process and high product purity. High effect with low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Add 0.2mmol cloxacillin to a 50mL ground-mouth Erlenmeyer flask with a stopper, and then add 10mL aqueous sodium hydroxide solution with a concentration of 0.2mol / L (the amount of solute sodium hydroxide is 2mmol) to obtain colorless Clarify the transparent solution, then put the Erlenmeyer flask into a heat-collecting magnetic stirrer, stir for 60 minutes at a speed of 500 r / min in a constant temperature water bath at 25°C for alkali destruction, and obtain the cloxacillin penicillin thiazole sodium intermediate;
[0035] 2) Add hydrochloric acid with a concentration of 0.1mol / L to the obtained cloxacillin penicillin thiazole sodium intermediate until the pH value is adjusted to 8.12, seal the Erlenmeyer flask and refrigerate at 4°C for 48 hours to obtain a slightly yellowish clarification Solution; the solution is taken out and freeze-dried immediately. The specific operation of freeze-drying is to freeze in a -80°C refrigerator for 12h, then transfer to a freeze dr...
Embodiment 2
[0037] 1) Add 0.2mmol cloxacillin to a 50mL ground-necked Erlenmeyer flask with a stopper, and then add 1mL aqueous sodium carbonate solution with a concentration of 2mol / L (the amount of solute sodium hydroxide is 2mmol) to obtain a colorless, clear and transparent solution, and then put the Erlenmeyer flask into a heat-collecting magnetic stirrer, stir at a speed of 600r / min for 5min in a constant temperature water bath at 60°C for alkali destruction, and obtain the cloxacillin penicillin thiazole sodium intermediate;
[0038] 2) Add hydrochloric acid at a concentration of 1 mol / L to the obtained cloxacillin penicillin thiazole sodium intermediate until the pH value is adjusted to 6, seal the Erlenmeyer flask and place it in a water bath at 30°C for a heating reaction for 24 hours, A slightly yellow clear solution was obtained; the solution was taken out and freeze-dried immediately. The specific operation of freeze-drying was to freeze in a -70°C refrigerator for 24 hours, a...
Embodiment 3
[0040] 1) Add 0.2mmol cloxacillin to a 50mL ground-mouth conical flask with a stopper, and then add 10mL of 0.02mol / L sodium hydroxide solution in absolute ethanol (the amount of solute sodium hydroxide is 0.2mmol ), to obtain a colorless, clear and transparent solution, and then put the Erlenmeyer flask into a heat-collecting magnetic stirrer, stir at a speed of 550r / min for 30min in a constant temperature water bath at 40°C for alkali destruction, and obtain cloxacillin penicillin thiazole Sodium acid intermediate;
[0041]2) Add nitric acid with a concentration of 0.01 mol / L to the obtained cloxacillin penicillin thiazole sodium intermediate until the pH value is adjusted to 10.2, seal the Erlenmeyer flask and place it in a water bath at 40°C for heating for 56 hours , to obtain a slightly yellowish clear solution; the solution was taken out and freeze-dried immediately. The specific operation of freeze-drying was to freeze in a -75°C refrigerator for 18h, then transfer to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com