Preparation method of cationic liposome SM-102 and analogue thereof
A technology of cationic liposomes and analogs, applied in the preparation of organic compounds, cyanide reaction preparation, carboxylate preparation, etc., can solve the problems of long time, difficult to control, and large pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Preparation of methyl 8-bromooctanoate (intermediate 1)
[0046] Add 600g of 8-bromooctanoic acid into a 3000mL reaction flask, add 2000ml of methanol, add 10g of concentrated sulfuric acid, heat at 50-65°C until the reaction is complete, cool to room temperature, add sodium carbonate to adjust to alkalinity, filter to remove insoluble solids, and recover methanol to obtain 8 -Methyl bromooctanoate 605g, yield 95%.
Embodiment 2
[0047] Example 2 Preparation of 8-bromooctanoic acid-9-heptadecanol ester (intermediate 2)
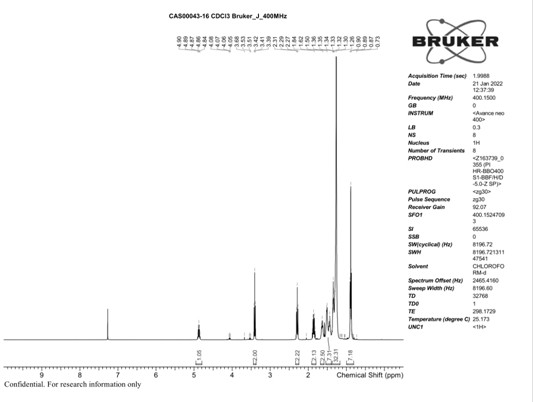
[0048] 600g of methyl 8-bromooctanoate was added into the 5000mL reaction flask, 650g of 9-heptadecanol was added, 2000ml of n-heptane as solvent, 10g of glycidol as a catalyst, heated to 80-90°C and stirred for 8h, and recovered after the reaction was completed. n-heptane to obtain 1165 g of colorless transparent liquid with a yield of 96%.
[0049] HNMR(300MHz,CDC1)δ:ppm4.89(m,1H);3.42(m,2H);2.31(m,2H);1.89(m,2H);1.73-1.18(m,36H);0.88(m , 6H).
Embodiment 3
[0050] Example 3 Preparation of 6-bromohexanoic acid-undecyl ester (intermediate 3)
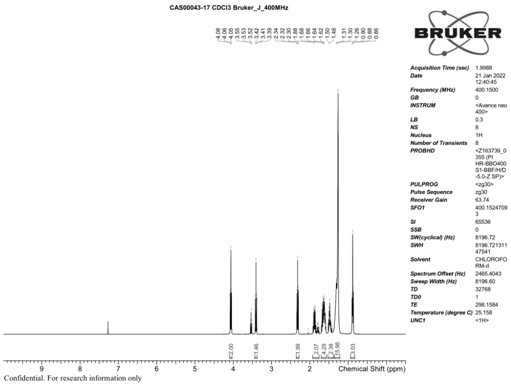
[0051] Add 1000g of 6-bromohexanoic acid, 885g of undecyl alcohol to the 3000L reaction flask, add 30g of catalyst potassium hydrogen sulfate, depressurize the water pump to the reaction pressure of 10-20mmHg, and heat to the inner temperature of 80-85°C at the same time, it can be seen that there is water evaporated, After about 5-6 hours of reaction, basically no water was evaporated, the reaction flask had a constant weight, cooled to room temperature, and about 100 g of silica gel was placed in a Buchner funnel, and the reaction solution was filtered to obtain 1718 g of a light yellow transparent liquid with a yield of 96%.
[0052] HNMR (300 MHz, CDC1 ) δ: ppm 4.06 (t, 2H); 3.40 (t, 2H); 2.29 (t, 2H); 1.85 (m,
[0053] 2H); 1.72-0.97 (m, 22H); 0.88 (m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com