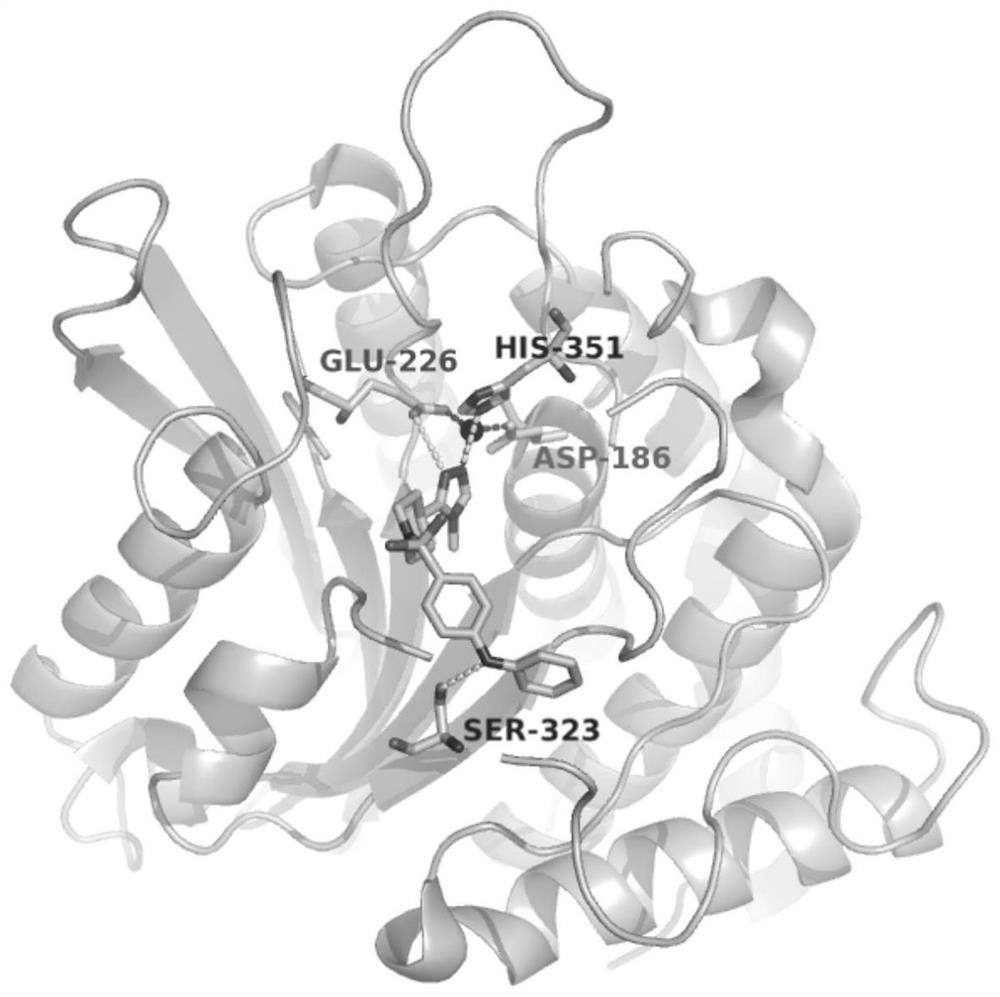
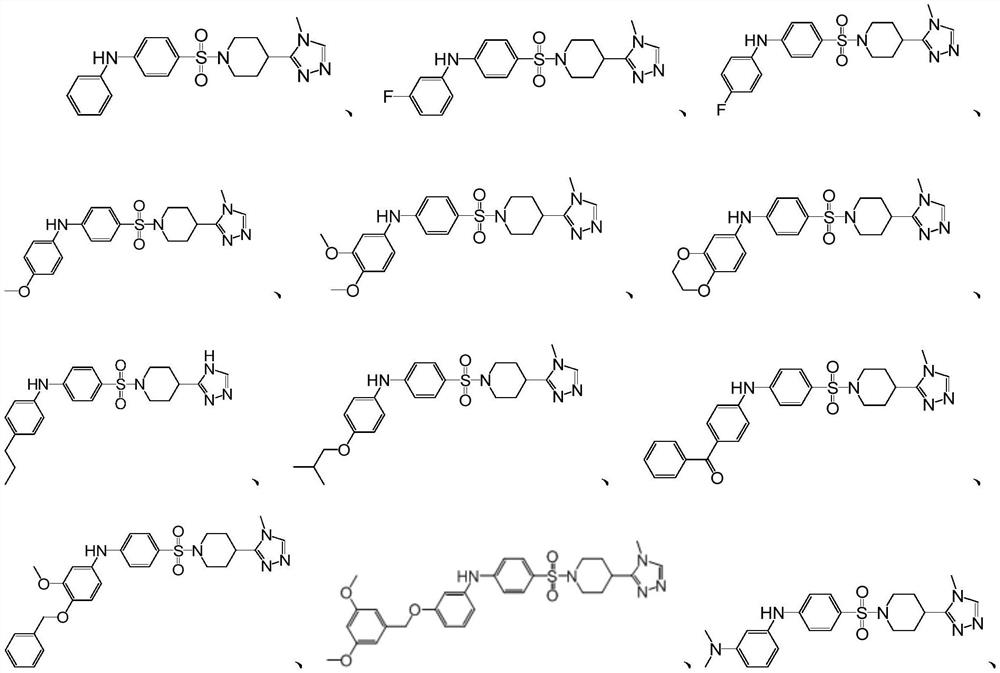
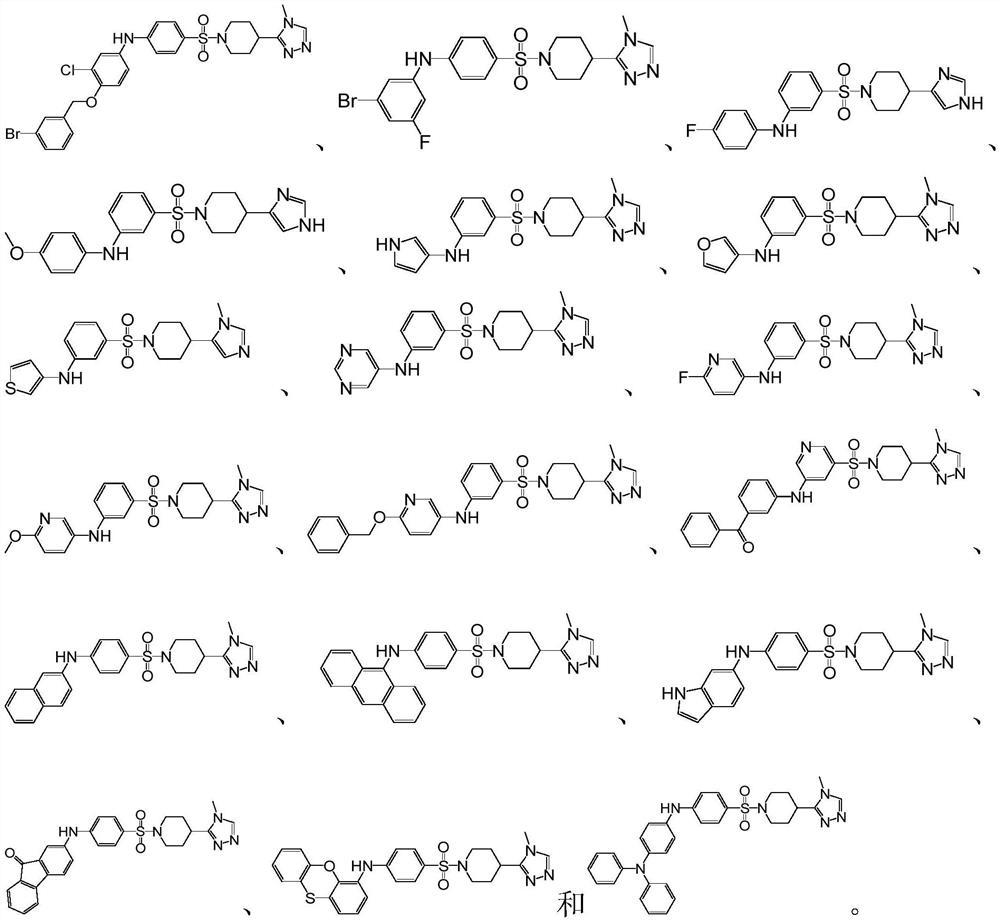
Glutamine acyl cyclase isoenzyme inhibitor as well as preparation method and application thereof
A technology of glutaminyl cyclase isoenzyme and inhibitor is applied in the field of glutaminyl cyclase isoenzyme inhibitor and preparation thereof, and can solve the problems of poor drugability of leading compounds, insufficient compounds and diverse molecular structures It can solve the problems of limited properties, etc., to achieve the effect of simple and feasible preparation method, easy availability of raw materials, and expansion of molecular structure diversity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] In some embodiments, also provide a kind of preparation method of glutaminyl cyclase isoenzyme inhibitor, it comprises the steps:
[0034] added to the reaction solvent Oxalyl chloride solution and DMF are reacted for the first predetermined time to obtain
[0035] Will And the triethylamine solution is added in the reaction solvent, and the reaction is carried out for the second predetermined time to obtain
[0036] added to the reaction solvent Copper acetate monohydrate and triethylamine solution are reacted for a third predetermined time to prepare the glutaminyl cyclase isoenzyme inhibitor.
[0037] In this embodiment, the The preparation includes the following steps: adding to the reaction solvent as well as Reaction for the fourth predetermined time, obtained Will Dissolve in the reaction solvent, add NaOH, react for the fifth predetermined time, add HCl, and prepare added to the reaction solvent AcOH and 30% hydrogen peroxide solution wer...
Embodiment 1
[0044] Preparation of 4-((4-(4-methyl-4H-1,2,4-triazol-3-yl)piperidin-1-yl)sulfonyl)-N-phenylaniline:
[0045] a. Add 10.05mmol of 4-(hydrazine carbonyl)piperidine-1-carboxylate tert-butyl ester and 12.03mmol of isothiocyanatomethane to 20mL of anhydrous EtOH, and reflux at 80°C for 3h. , and dried to obtain a white solid which is 4-(2-(methylaminomethylthio)hydrazine-1-carbonyl)piperidine-1-carboxylic acid tert-butyl ester with a yield of 97%.
[0046] b. 9.75mmol of 4-(2-(methylaminomethylthio)hydrazine-1-carbonyl)piperidine-1-carboxylate tert-butyl ester and 19.57mmol of NaOH were added to 20mL of water, and the reaction was carried out under reflux at 100°C for 2h. Let stand for cooling, add 4 mL of concentrated hydrochloric acid dropwise, stir at room temperature for 10 min, suction filtration, and dry the obtained white solid, which is 4-(4-methyl-5-sulfoxy-4,5-dihydro-1H-1,2, 4-Triazol-3-yl)piperidine-1-carboxylate tert-butyl ester, 85% yield.
[0047] c. 20mL CH 2 C...
Embodiment 2
[0053] 3,4-Dimethoxy-N-(4-((4-(4-methyl-4H-1,2,4-triazol-3-yl)piperidin-1-yl)sulfonyl)benzene Base) the preparation of aniline:
[0054] a. Add 10.01 mmol of 4-(hydrazine carbonyl) piperidine-1-carboxylic acid tert-butyl ester and 12.04 mmol of isothiocyanatomethane to 20 mL of anhydrous EtOH, and react at 80° C. for 4 h under reflux. , and dried to obtain a white solid which is 4-(2-(methylaminomethylthio)hydrazine-1-carbonyl)piperidine-1-carboxylic acid tert-butyl ester with a yield of 98%.
[0055] b. 9.81mmol of 4-(2-(methylaminomethylthio)hydrazine-1-carbonyl)piperidine-1-carboxylate tert-butyl ester and 20.23mmol of NaOH were added to 20mL of water, and the reaction was performed under reflux at 100°C for 2h. Let stand for cooling, add 4 mL of concentrated hydrochloric acid dropwise, stir at room temperature for 10 min, suction filtration, and dry the obtained white solid, which is 4-(4-methyl-5-sulfoxy-4,5-dihydro-1H-1,2, 4-Triazol-3-yl)piperidine-1-carboxylate tert-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com