Method for synthesizing 5-hydroxy beta-indolyl alanine by taking beta-indolyl alanine as substrate and application of 5-hydroxy beta-indolyl alanine
A technology of indolylalanine and indolylalanine protein, which is applied in the biological field, can solve the problems of high price and difficult storage of BH4, and achieve the effect of high synthesis yield and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Construction and screening of single BH4 regeneration system engineering bacteria
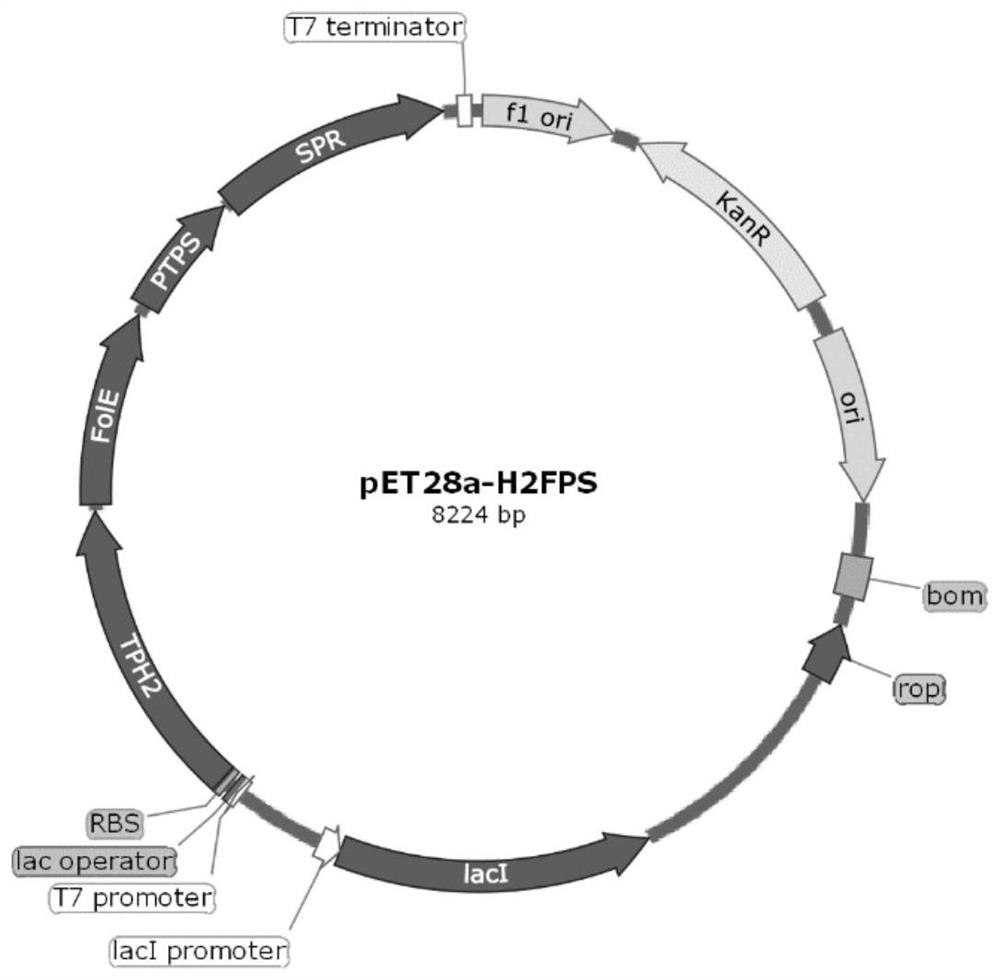
[0047] Tandem expression of key enzyme gene TPH2 in β-indolylalanine hydroxylation pathway; key enzyme FOLE, PTPS and SPR in BH4 synthesis pathway.
[0048] The TPH2, FOLE, PTPS and SPR genes were synthesized and inserted between the NdeI and XhoI sites of the vector plasmid pET28a(+) to obtain pET28a-TPH2, pET28a-FOLE, pET28a-PTPS and pET28a-SPR plasmids.
[0049] Using F1 and R1 as primers, the pET28a-TPH2 plasmid was used as the template to clone the pET28a plasmid vector containing the TPH2 gene; using F2 and R2 as the primers, the plasmid pET28a-FOLE was used as the template to clone to obtain the FOLE gene; using F3 and R3 as the primers, the plasmid pET28a was cloned - PTPS was used as template to clone to obtain PTPS gene; F4 and R4 were used as primers, plasmid pET28a-SPR was used as template to clone to obtain SPR gene.
[0050] The above fragments were ligated toget...
Embodiment 2
[0066] Embodiment 2 Construction and screening of double BH4 regeneration system engineering bacteria
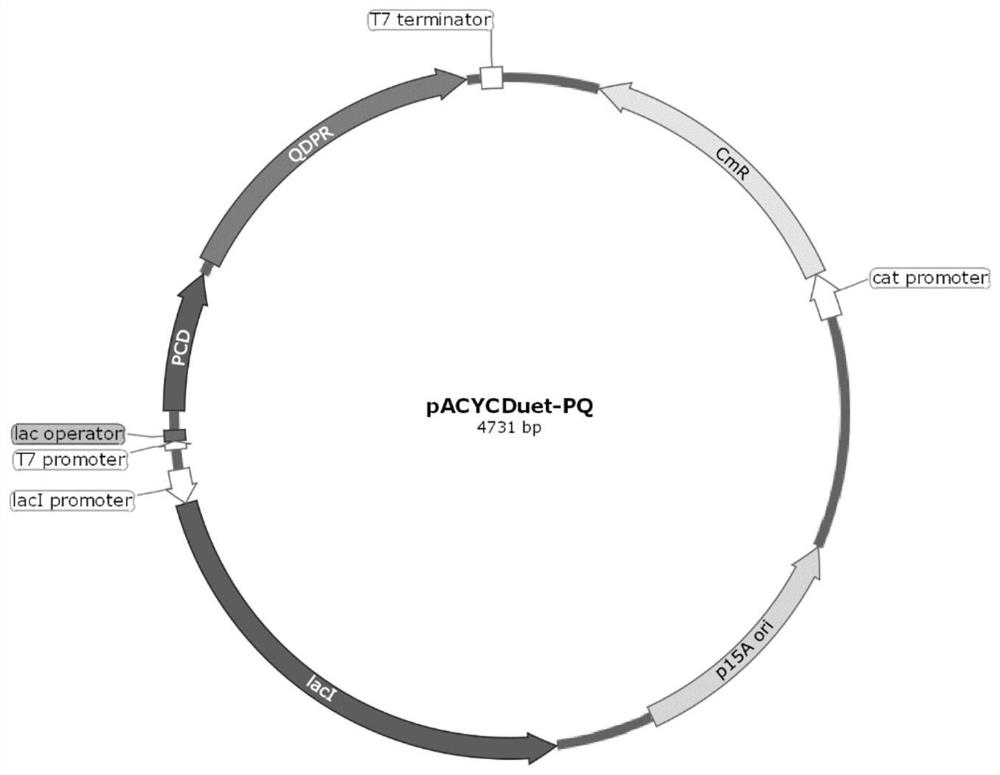
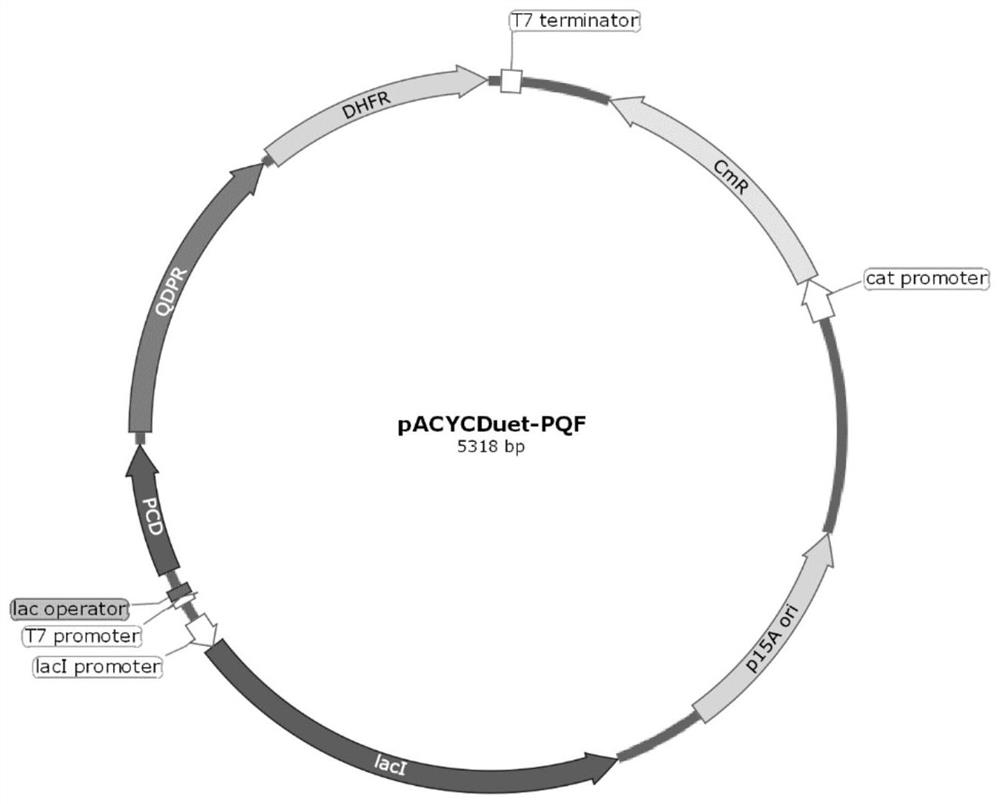
[0067] The above-mentioned BH4 regeneration key genes QDPR and DHFR were further combined to form a double BH4 regeneration system, and the effect of screening the engineering bacteria of these different gene combinations to synthesize 5-hydroxyβ-indolylalanine was evaluated. Specifically, a double BH4 regeneration system was constructed by taking the PCD gene shown in SEQ ID NO: 5 and the QDPR gene shown in SEQ ID NO: 8 as examples.
[0068] Based on the double BH4 regeneration system, the synthesis route of 5-hydroxyβ-indolylalanine using β-indolylalanine as substrate is as follows: Figure 4 shown.
[0069] Tandem expression of key genes PCD, QDPR and different DHFRs in the dual BH4 regeneration system:
[0070] DHFR genes from different sources were selected and synthesized by codon optimization to obtain F1-F5 (the sequences are shown in SEQ ID NOs: 11-15 in sequence)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com