Method for the production of polypeptides
A technology of polypeptide chains and heterologous polypeptides, applied in the direction of microorganism-based methods, biochemical equipment and methods, fusion polypeptides, etc., can solve problems such as proteolytic degradation sensitivity, unstable structure, and heterogeneous products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Disruption of the Δyap3::URA3 gene
[0057] The Δura3 deletion mutation was constructed as follows:
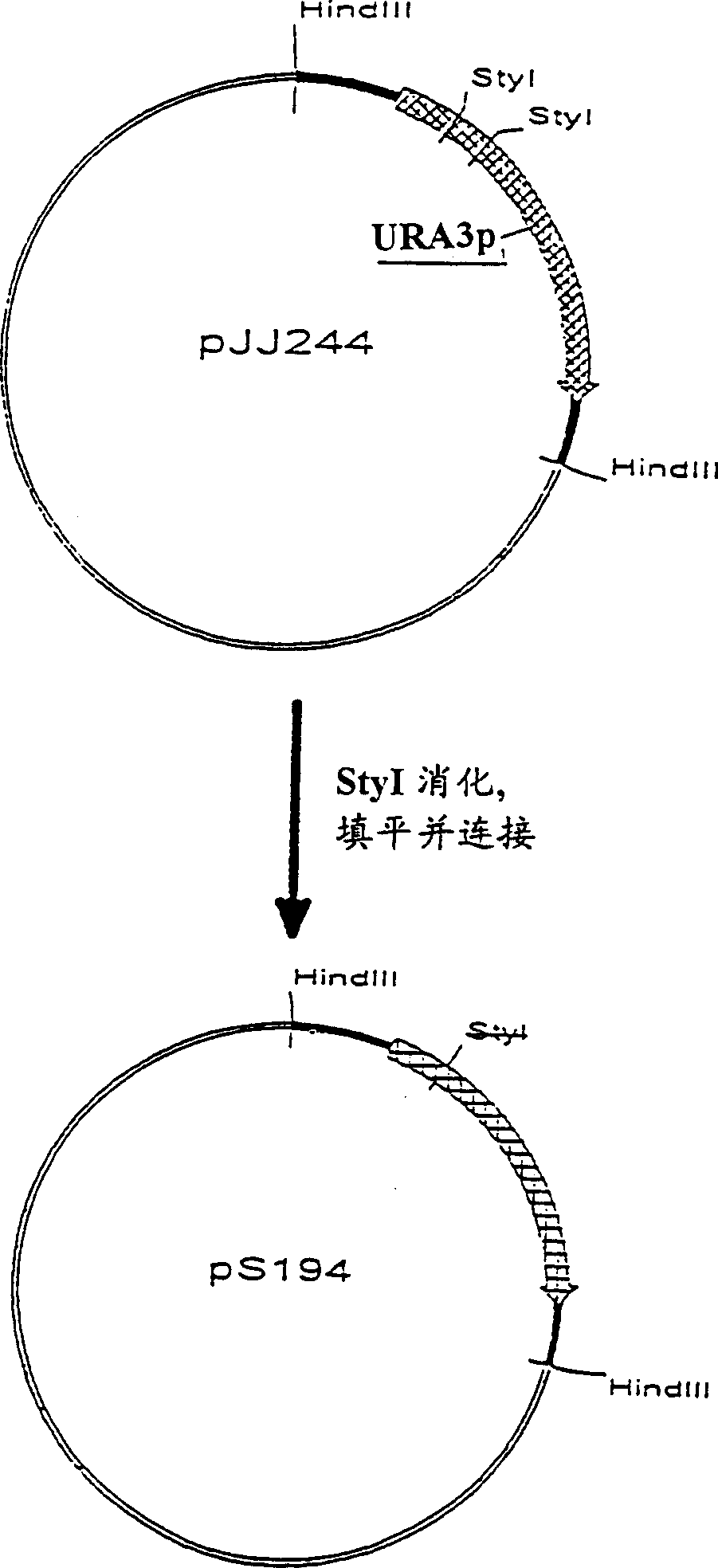
[0058] pJJ244 (pUC18 including the 1.2 kb Hind III fragment of the URA3 gene) was digested with StyI, filled in with Klenow polymerase, and self-ligated. The resulting plasmid, called pS194, contains the URA gene with the 84 bp Sty I-Sty I fragment deleted, see figure 1 .
[0059] Δyap3::URA3 gene disruption plasmid pME1389 was constructed as follows:
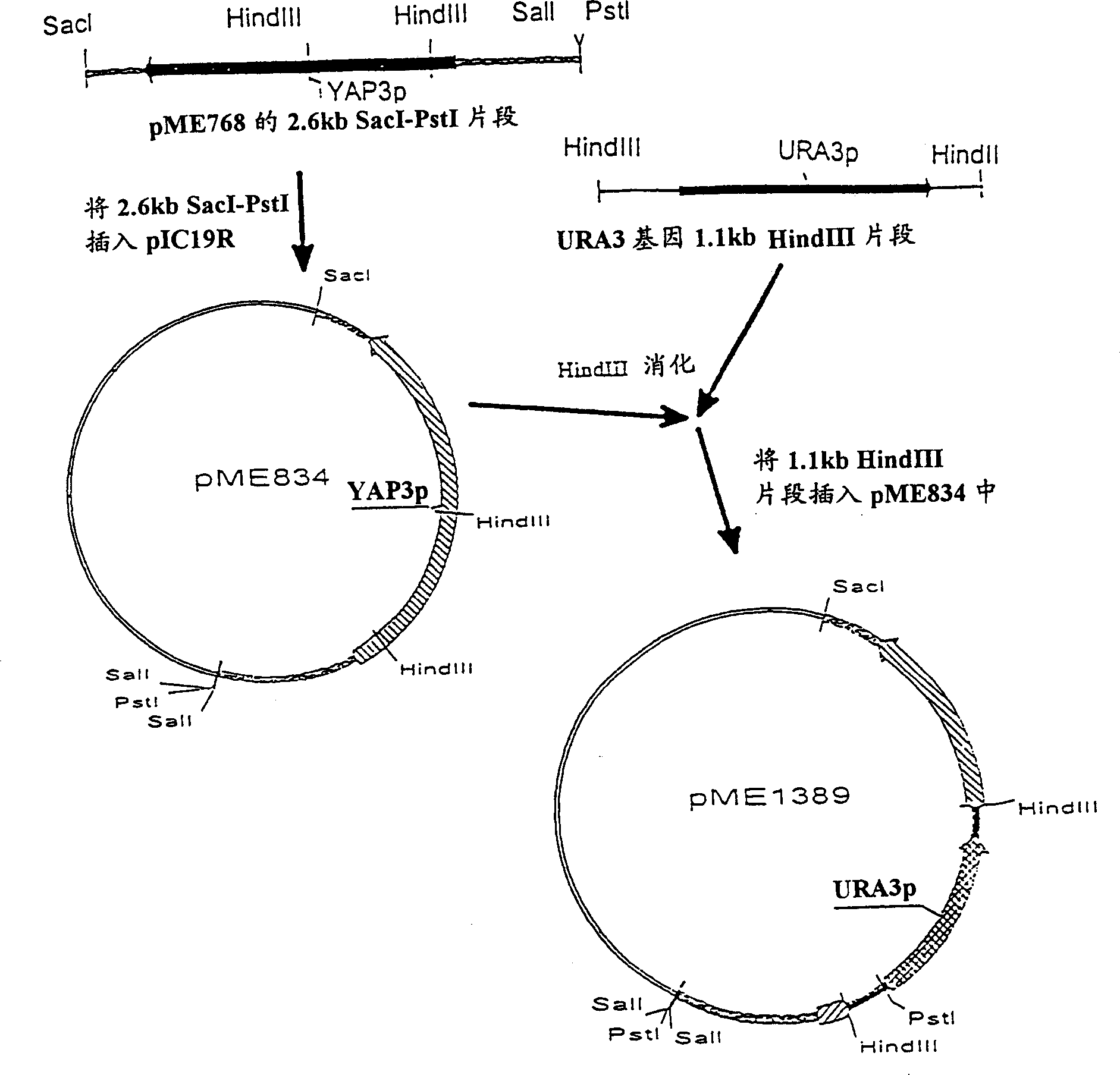
[0060] The 2.6 kb Sac I-Pst I fragment containing the YAP3 gene in pME768 (Egel-Mitani et al., Yeast 6: 127-137, 1990) was inserted into the 2-6 kb Sac I fragment of pIC19R (Marsh et al., Gene 32: 481-485, 1984). In the l-Pst I fragment, the resulting plasmid was pME834. pME834 was digested with Hind III to delete the 0.7 kb YAP3 fragment and replace it with the 1.2 kb Hind III fragment inserted into the URA3 gene (Rose et al., Gene, 29:113-124, 1984), resulting in the plasmid pME1389. The construction of...
Embodiment 2
[0063] Embodiment 2: Construction of diploid Δyap3 / Δyap3 strain
[0064] ME1487 was mutagenized with EMS (ethyl methanesulfonate), and ura3 mutants were selected on plates containing 5-FOA. and then by using Image 6 One of the selected isolates, ME1656, was transiently transformed with pME973 as shown to undergo a mating type switch (Herskowitz and Jensen, Methods in Enzymology, 194:132-146, 1991). pME973 contains the genes encoding the HO (homophylic complex) endonuclease and URA3 inserted into the YEp13 plasmid (Rose and Broach, Methods in Enzymology, 185:234-279, 1990). Haploid strain ME1695, which had switched from MATα to MATa and had the following genetic background: MATa Δyap3::ura3 pep4-3Δtpi::LEU2 leu2Δura3, was selected from transient transformants.
[0065] ME1695 was then hybridized to ME1487 by micromanipulation (Sherman and Hicks, Methods in Enzymology, 194: 21-37, 1991) to obtain Δyap3 / Δyap3 diploids. From the resulting diploids, line ME1719 was selected wit...
Embodiment 3
[0067] Construction of Δyap3::URA3::Δylr121c Double Disruption Strain
[0068] To construct a one-step gene disruption strain of two tightly linked genes encoding YAP3 and YLR121C, the following two oligonucleotide primers were synthesized:
[0069] P1 length 57bp: YLR121C / URA 3 primer
[0070] 5′-GAT CGA ACG GCC ATG AAA AAT TTG TAC TAG CTA ACG
[0071] AGC AAA GCT TTT CAA TTC AAT-3′
[0072] P2 length 57bp: YAP3 / URA3 primer
[0073] 5′-CCA GAA TTT TTC AAT ACA ATG GGG AAG TTG TCG TAT
[0074] TTA TAA GCT TTT TCT TTC CAA-3′
[0075] P1 and P2 each contain 40 nucleotides corresponding to the sequences in the YLR121C and YAP3 coding regions, and also contain a Hind III restriction site (AAGCTT) and 12 nucleotides corresponding to the flanking sequence of the URA3 gene (YEL021W) acid. Using URA3 gene as a template, PCR was carried out using P1 and P2, and the generated 1248bp PCR fragment contained a URA3 selectable marker, which was flanked by 40 nucleotides derived...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com