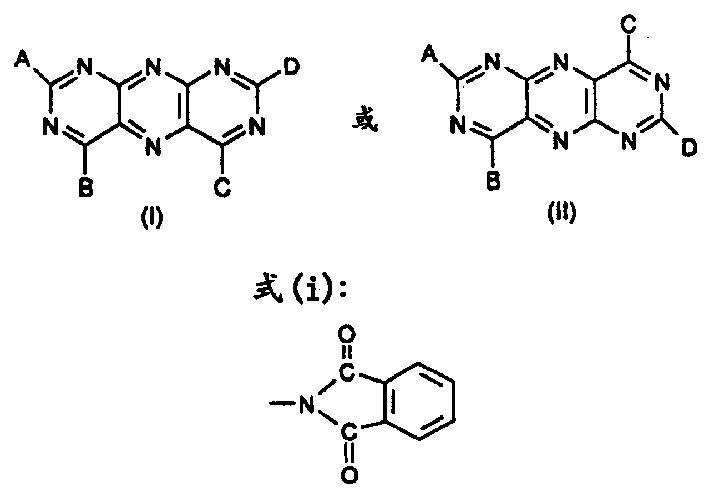
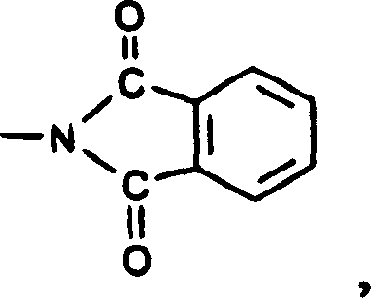
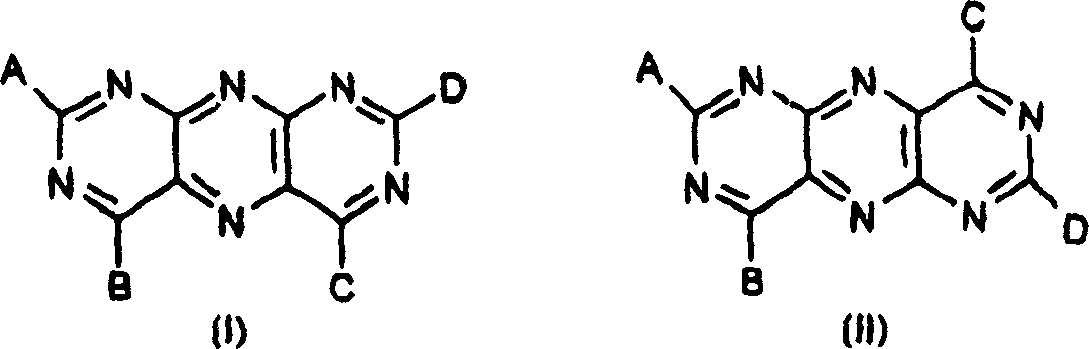
Bulk coloured high polymer organic material containing pyrimido-pteridine or its mixture
A high molecular weight, organic material technology, applied in the direction of organic dyes, organic chemistry, dyeing methods, etc., can solve the problem of not being particularly excellent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 : 198.8 g of commercially available 90% 2,4,5,6-tetraaminopyrimidine sulfate was slurried in 1 l of deionized water, then the suspension was adjusted to pH 7.9 by adding 30% aqueous sodium hydroxide solution ( measured with a pH glass electrode). At the beginning of the oxidation step, the pH was maintained at 7.9 and, if necessary, 30% aqueous sodium hydroxide was added. The reaction mixture was first stirred at room temperature for 15 minutes and then heated to 55°C over 30 minutes. Moderate airflow was then blown through the subsurface tube into the then almost clear orange solution, raising the temperature to 85°C over a further 30 minutes. The gradually thickening orange suspension was then stirred at 85° C. for 20 hours while blowing in air. After 3 hours, 150 ml of water were added and the pH control was stopped (total consumption of 30% sodium hydroxide solution: 197 ml). The fine orange precipitate was isolated hot by filtration through a hard fil...
Embodiment 2
[0071] Example 2 : The filtrate containing 10 l of still warm glacial acetic acid mentioned in Example 1 was left overnight at room temperature. The resulting fine precipitate was isolated by filtration through a hard filter paper, and the orange-red residue was stirred in 600 ml of water. This suspension, having a pH value of 4.37, was adjusted to pH 7 with 30% sodium hydroxide solution, then heated to 90° C. and adjusted to pH 7 again by further addition of sodium hydroxide solution. The suspension was stirred for one hour at 90°C, then filtered hot through a hard filter paper and the filter cake was washed with 1200 ml of water. While still moist, the orange-red filter cake was slurried in 600 ml dimethylacetamide and heated to boiling point, first distilling off the solvent / water mixture until the temperature of the suspension reached 140°C. At 140° C., the suspension was recrystallized for 17 hours, the reaction product was cooled to 90° C., filtered through a hard fil...
Embodiment 3
[0075] Example 3: 24.66 g of commercially available 97% 6-hydroxy-2,4,5,-triaminopyrimidine sulfate was slurried in 450 ml of deionized water, and the suspension was adjusted to pH by adding 30% aqueous sodium hydroxide The value is 7.5 (measured with a pH glass electrode). The mixture was heated to 80° C. within 30 minutes and the pH was adjusted continuously to 7.5 by addition of sodium hydroxide solution. A moderate air stream was then blown through the subsurface tube into the tan suspension, maintaining the temperature at 80°C throughout. After 22 hours, the gas flow was stopped and the orange suspension was cooled to 60°C and filtered through a hard filter paper. After washing with 150 ml of water, the still damp filter cake was mixed with 300 ml of glacial acetic acid (laboratory mixer) and the suspension was then placed in a Soxhlet tube and extracted with a further 1200 ml of boiling glacial acetic acid for 2 hours. The orange residue was collected by suction from...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com