Nucleosides with anti-hepatitis B virus activity
A compound, acyl technology, applied in the field of treatment of hepatitis B virus, can solve the problem of not being very effective, nucleotides can not effectively pass through the cell membrane, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
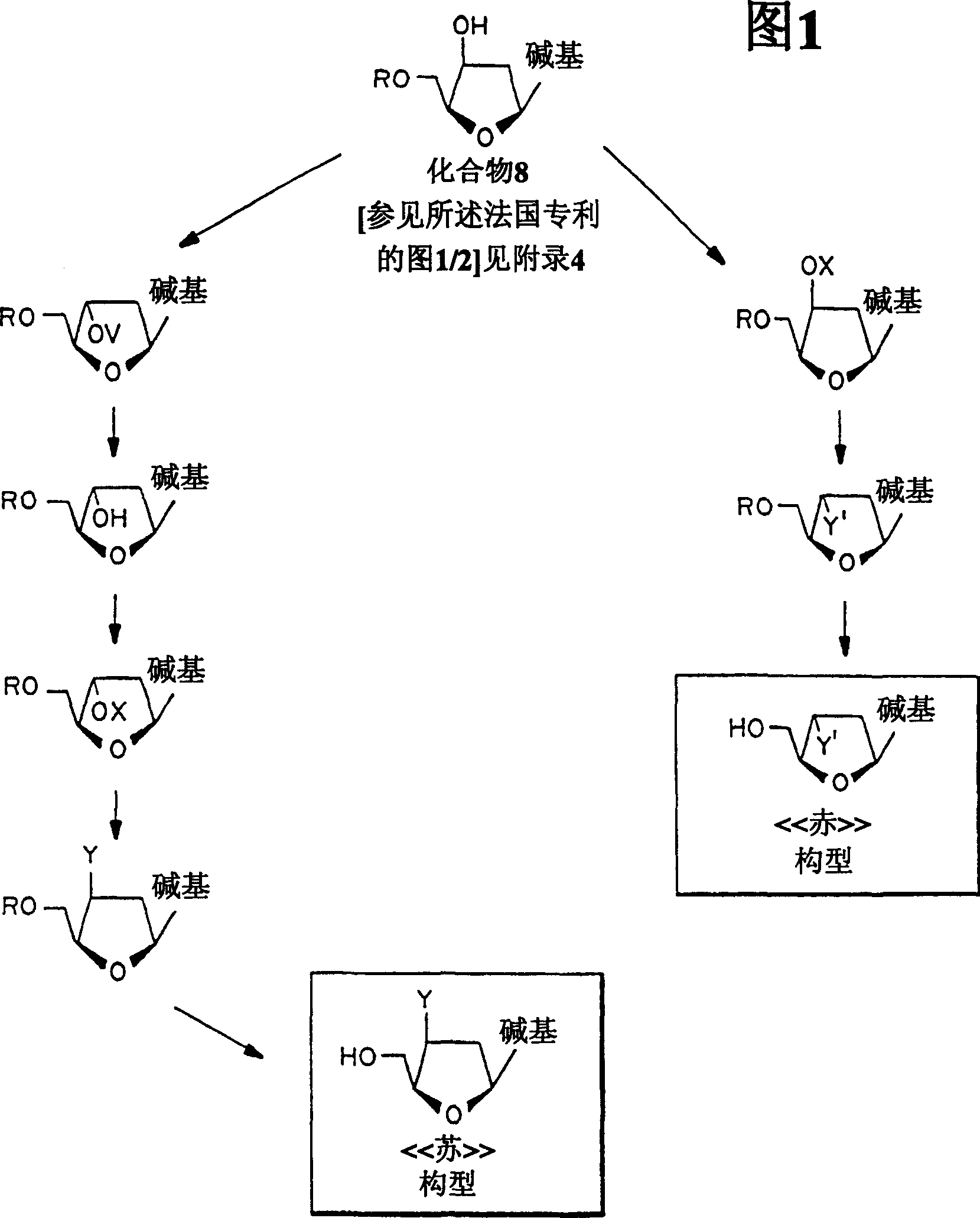
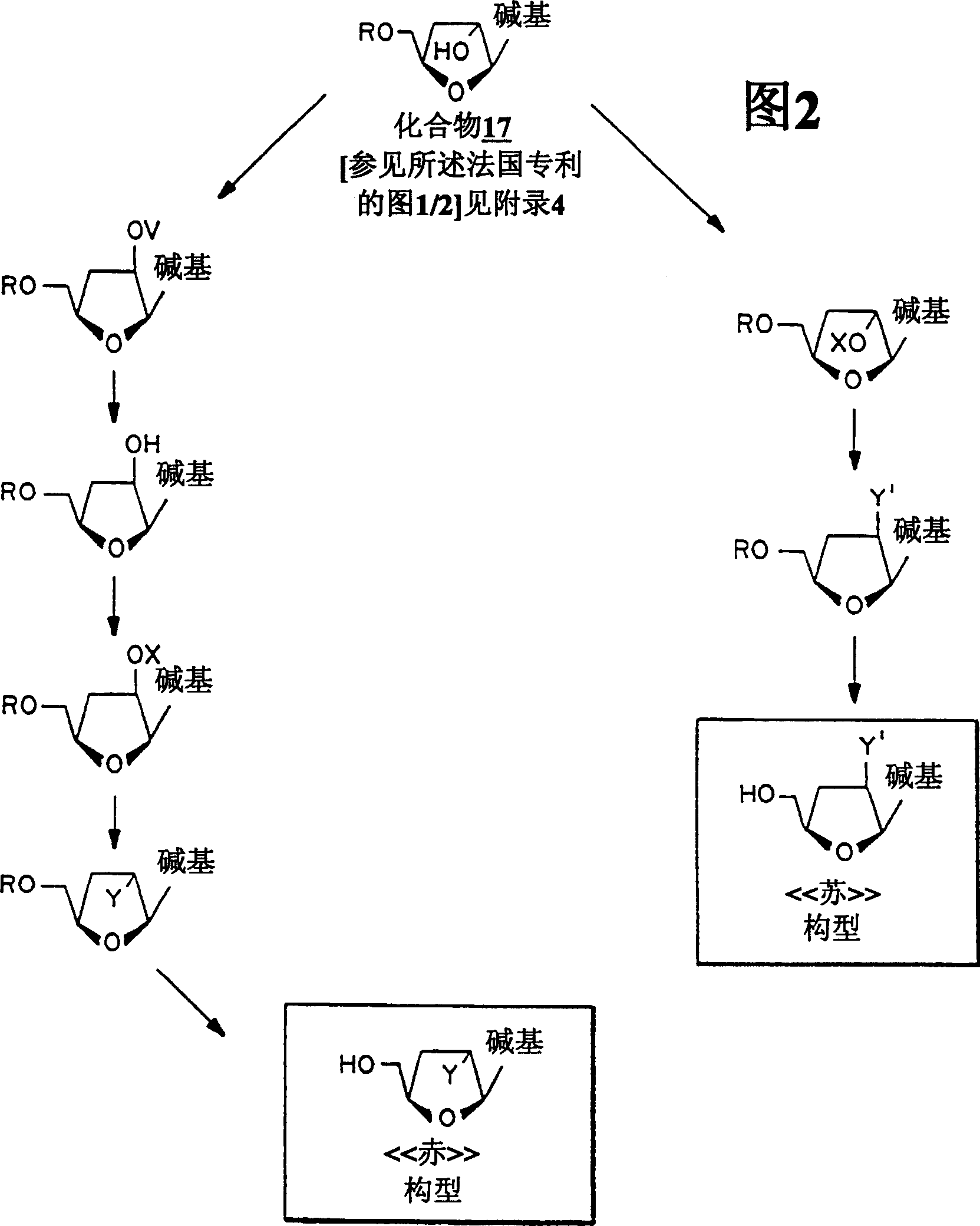
Image
Examples
Embodiment 1
[0068] Preparation of β-L-(3'-azido)-2',3'-dideoxy-5-fluorocytosine
[0069] Melting points were determined in open capillary tubes with a Gallenkamp MFB-595-010 M instrument and were uncorrected. UV absorption spectrum was measured in ethanol with Uvikon931 (KONTRON) spectrophotometer. 1 H-NMR at room temperature in DMSO-d 6 Measured with Bruker AC 250 or 400. Chemical shifts are expressed in ppm, DMSO-d 6 Set at 2.49ppm as a reference. Perform deuterium exchange, decoupling experiments or 2D-COSY to confirm the arrangement of hydrogen nuclei, and the multiplicity of signals is represented by s (singlet), d (doublet), dd (doublet doublet), t (triplet), q (quartet), br (broad), m (multiplet), and all J values are expressed in Hz. FAB mass spectra were recorded with a JEOL DX300 mass spectrometer in positive (FAB>0) or negative (FAB-1 deg cm 2 g -1 express. Elemental analyzes were performed according to the "Service de Microanalyses du CNRS, Division de Vernaison" (...
Embodiment 2
[0100] Example 2 Preparation of β-L-(2'-azido)-2',3'-dideoxy-5-fluorocytosine
[0101] The general procedure and apparatus used have been described in Example 1 for the synthesis of the 3'-isomer (3'-N 3 - Experimental recording part of β-L-FddC).
[0102] 1-(2-O-acetyl-3-deoxy-5-O-benzoyl-β-L-erythro-pentofuranosyl)-5-fluorouracil 13
[0103]
[0104] A suspension of 5-fluorouracil (5.15 g, 39.6 mmol) was treated with hexamethyldisilazane (HMDS, 257 mL) and a catalytic amount of ammonium sulfate and refluxed for 18 hours. After cooling to room temperature, the mixture was evaporated under reduced pressure to give a residue as a colorless oil which was diluted with anhydrous 1,2-dichloroethane (290ml). To the resulting solution was added 1,2-di-O-acetyl-3-deoxy-5-O-benzoyl-L-erythro-pentofuranose in dry 1,2-dichloroethane 12 (8.5g, 26.4mmo) [Reference: Mathe, C., Ph.D. Dissertation, Universite de Montpellier II-Sciences et Techniques du Languedoc, Montpellier (France), ...
Embodiment 3
[0146] Example 3 Toxicity of compounds
[0147] The ability of the active compounds to inhibit virus growth in 2.2.15 cell culture (HepG2 cells transformed with hepatitis virions) was evaluated. As can be seen from Table 1, no overt toxicity (greater than 50% inhibition of the level of dye uptake observed in untreated cells) was observed for any of the compounds tested at a concentration of 100 mM.
[0148] Toxicity assays were performed on 96-well flat-bottomed tissue culture plates. Cells for toxicity analysis were cultured and treated with test compounds in the same manner as for antiviral evaluation. Four concentrations of each compound were tested in triplicate cultures, and relative levels of toxicity were determined using neutral red dye uptake. The absorption of the dye that will enter the interior at 510nM (A 510 ) for quantitative analysis. Average A of 9 independent cultures of untreated cells kept in the same 96-well plate 510 Quantitative analysis results wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com