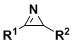
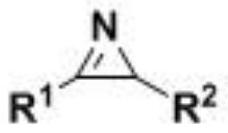
A kind of synthetic method of α-amino acid derivatives
A synthetic method and technology of amino acids, applied in the field of organic synthesis methodology, can solve the problems of unreported disconnection of carbon-nitrogen double bonds, etc., and achieve the effect of simple reaction conditions and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The reaction formula of the whole process is as follows:
[0024]
[0025]
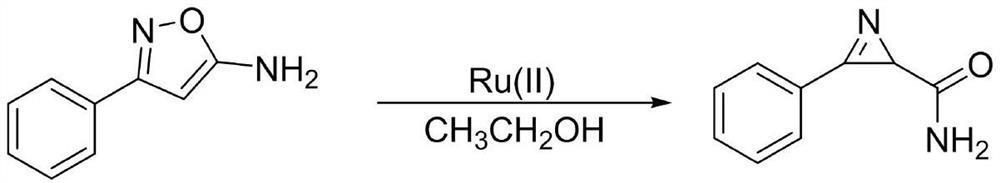
[0026] The preparation method of the aziridine compound with amide: add 1.0 equiv. (0.2 mmol) of the oxazole ring to the Shrek tube, add 2 mL of analytical pure ethanol, 1% equiv. of ruthenium catalyst, under the condition of green light Under reaction for 24-72 hours. After the reaction, spin off the solvent with a rotary evaporator, extract the organic phase three times with water and dichloromethane, dry the organic phase with anhydrous sodium sulfate, spin off the excess solvent, and then pass through column chromatography (dichloromethane / methanol) to obtain Aziridines with amides.
[0027] Synthesis of α-amino acid derivatives (diphenylthiopropionamide compound): add 1.0 equiv. (0.2 mmol) of the above-mentioned aziridine compound with amide to the Shrek tube, 1.5 mL of analytically pure methanol, and 1.5 mL of pH 7.40 buffer solution (potassium dihydrogen phosphate-dipotassium hyd...
Embodiment 2
[0029] The reaction formula of the whole process is as follows:
[0030]
[0031] The preparation method of the aziridine compound with ester group: add 1.0equiv (0.2mmol) of p-toluenesulfonimide derivative to the Shrek tube, add 2mL of analytically pure dichloromethane, 1.5equiv (0.3mmol) Triethylamine was stirred and reacted in an ice-water bath for 6 hours, and the reaction stopped when the temperature returned to room temperature. Extract the organic phase with water and dichloromethane three times, dry the organic phase with anhydrous sodium sulfate, spin off more than the solvent, and then pass through column chromatography (petroleum ether / ethyl acetate) to obtain the aziridine compound with ester group .
[0032] Synthesis of α-amino acid compounds (diphenylthiopropylamine tert-butyl ester compound): Add 1.0equiv (0.2mmol) of the above-mentioned aziridine compound with ester group to the Shrek tube, 1.5mL analytical pure methanol, 1.5mLpH The buffer solution (pota...
Embodiment 3
[0034] The reaction formula of the whole process is as follows:
[0035]
[0036] The preparation method of 3-phenylaziridine compound: add 1.0 equiv (0.2 mmol) of 1-azidovinylbenzene derivative to a round bottom flask, add 10 mL of toluene, and heat at reflux at 110 ° C for 6 hours . After the reaction is finished, spin off the excess solvent, and then go through column chromatography (petroleum ether / ethyl acetate) to obtain the 3-phenylaziridine compound.
[0037] Synthesis of α-amino acid compounds (diphenylthiopropylamine compound): Add 1.0 equiv (0.2 mmol) of the above-mentioned 3-phenylazacyclopropene compound to the Shrek tube, 1.5 mL of analytically pure methanol, and 1.5 mL of pH value is 7.40 The buffer solution (potassium dihydrogen phosphate-dipotassium hydrogen phosphate) was vacuumized, and 8.0 equiv of thiophenol was added dropwise to the Shrek tube to react for 20 hours under the condition of blowing nitrogen. After the reaction was completed, the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com