Antifibrosis pyridinone compound and its preparing process
A technology for synthesizing pyridones and compounds, which is applied in the field of synthesis of pyridone compounds and improvement of synthetic process methods, can solve the problems of easy polymerization, difficult to obtain compounds, unstable compounds and the like, and achieves simple and easily controllable reaction process and wide adaptability. The effect of sexual and structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
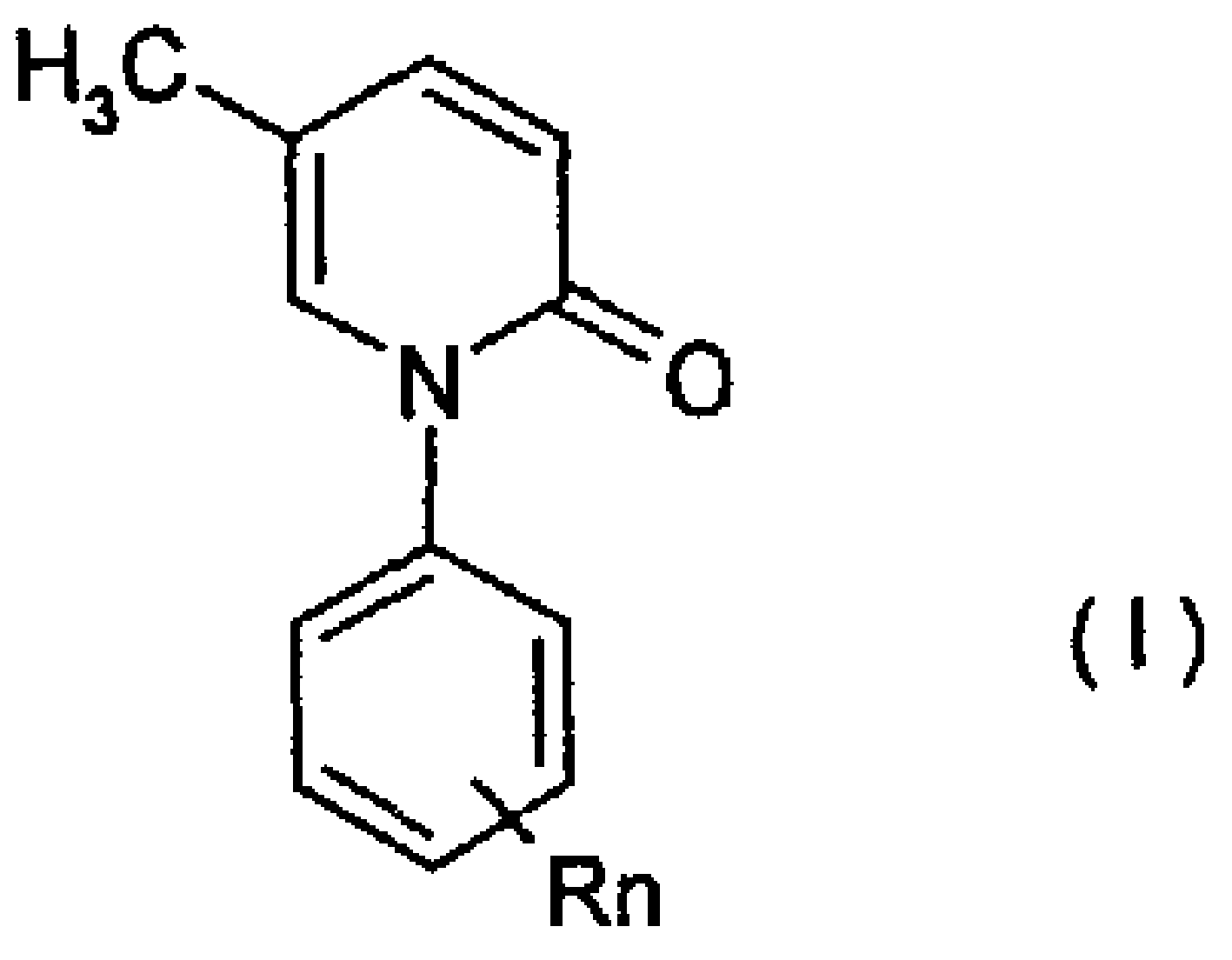
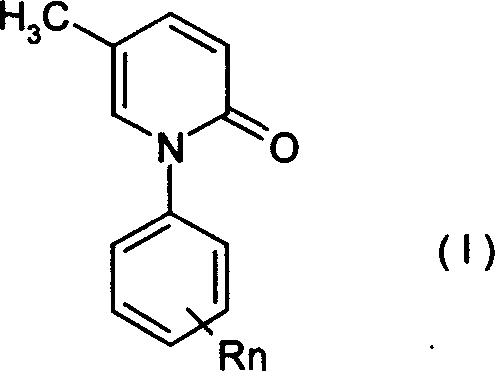
[0037] In the 1-substituted phenyl-5-methyl-2(1H)pyridone of formula (I), n=1, R=Br, such as:
[0038] 1-(2-Bromophenyl)-5-methyl-2-(1H)pyridone;
[0039] 1-(3-Bromophenyl)-5-methyl-2-(1H)pyridone;
[0040] 1-(4-Bromophenyl)-5-methyl-2-(1H)pyridinone.
Embodiment 2
[0042] In the 1-substituted phenyl-5-methyl-2(1H)pyridone of formula (I), n=2, R=Br or Cl, such as:
[0043] 1-(2,3-Dibromophenyl)-5-methyl-2-(1H)pyridone
[0044] 1-(2,4-Dibromophenyl)-5-methyl-2-(1H)pyridone
[0045] 1-(2,5-Dibromophenyl)-5-methyl-2-(1H)pyridone
[0046] 1-(2,6-Dibromophenyl)-5-methyl-2-(1H)pyridone
[0047] 1-(3,4-Dibromophenyl)-5-methyl-2-(1H)pyridone
[0048] 1-(3,5-Dibromophenyl)-5-methyl-2-(1H)pyridone
[0049] 1-(2,3-Dichlorophenyl)-5-methyl-2-(1H)pyridone
[0050] 1-(2,4-Dichlorophenyl)-5-methyl-2-(1H)pyridone
[0051] 1-(2,5-Dichlorophenyl)-5-methyl-2-(1H)pyridone
[0052] 1-(2,6-Dichlorophenyl)-5-methyl-2-(1H)pyridone
[0053] 1-(3,5-Dichlorophenyl)-5-methyl-2-(1H)pyridone
Embodiment 3
[0055] In the 1-substituted phenyl-5-methyl-2(1H)pyridone of formula (I), n=1, R=trifluoromethyl, such as:
[0056] 1-(2-Trifluoromethylphenyl)-5-methyl-2-(1H)pyridone
[0057] 1-(4-Trifluoromethylphenyl)-5-methyl-2-(1H)pyridone
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com