Tabletted prepn.
A technology of composition and tablet, which is applied in the field of tablet composition and can solve the problems of reduced bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Each component shown in Table 1 was weighed, and each component except magnesium stearate was mixed for 10 minutes with the stirring granulator. Next, add pure water (15-75 parts by weight) that can produce particles with a particle diameter of about 100-500 μm, and perform stirring and granulation for 10 minutes. All granules were crushed with a crusher and dried. Add magnesium stearate to the obtained dry granules, mix with a V-type mixer for 2 minutes, and compress to form a tablet with a diameter of 7 mm, a thickness of 3 mm, and a weight of 100 mg. The disintegration time of the obtained tablet in water was tested according to the disintegration test method of the Japanese Pharmacopoeia. Also, L-HPC (LH-31) [10 to 12.9% by weight of hydroxypropoxyl group, average particle diameter of 30 μm or less] manufactured by Shin-Etsu Chemical Co., Ltd. was used as low-substituted hydroxypropyl cellulose. The results are shown in Table 1.
[0024] Comparative pr...
Embodiment 2
[0026] 250 g of compound [1], 530 g of lactose, and 200 g of low-substituted hydroxypropyl cellulose [LH-31 manufactured by Shin-Etsu Chemical Co., Ltd., 10.0 to 13.0% by weight of hydroxypropoxy, and an average particle size of 30 μm or less) , after fully stirring in the stirring and mixing granulator, add 10 grams of hydroxypropyl cellulose [Nippon Soda (KK) HPC-L] dissolved in 500 grams of pure water and carry out stirring and granulation. After the obtained granules were crushed and dried, 10 grams of magnesium stearate was added and tableted to obtain a tablet with a diameter of 7 mm, a thickness of 3.7 mm, a weight of 120 mg, and a tablet containing 30 mg of compound [1]. The tablet was sprayed with a coating liquid composed of 8 g of hydroxypropyl methylcellulose, 1.5 g of polyethylene glycol 6000, 2.4 g of talc, 0.5 g of titanium oxide and 87.6 g of pure water to obtain coated tablets. Comparative example 1
Embodiment 3
[0032] 330 grams of compound [1], 450 grams of lactose and 200 grams of oligomeric hydroxypropyl cellulose [LH-31 produced by Shin-Etsu Chemical Industry Co., Ltd.] were fully stirred in a mixing granulator, and then added and dissolved in 500 10 grams of hydroxypropyl cellulose [Nippon Soda (KK) HPC-L] in gram pure water and carry out stirring granulation. After the obtained granules were crushed and dried, 10 grams of magnesium stearate was added and tabletted to obtain a tablet with a diameter of 7 mm, a thickness of 3.7 mm, a weight of 121 mg, and a tablet containing 40 mg of compound [1]. The tablet was sprayed with a coating liquid composed of 8 grams of hydroxypropyl methylcellulose, 1.5 grams of polyethylene glycol 6000, 2.4 grams of talc, 0.5 grams of titanium oxide and 87.6 grams of pure water to obtain coated tablets.
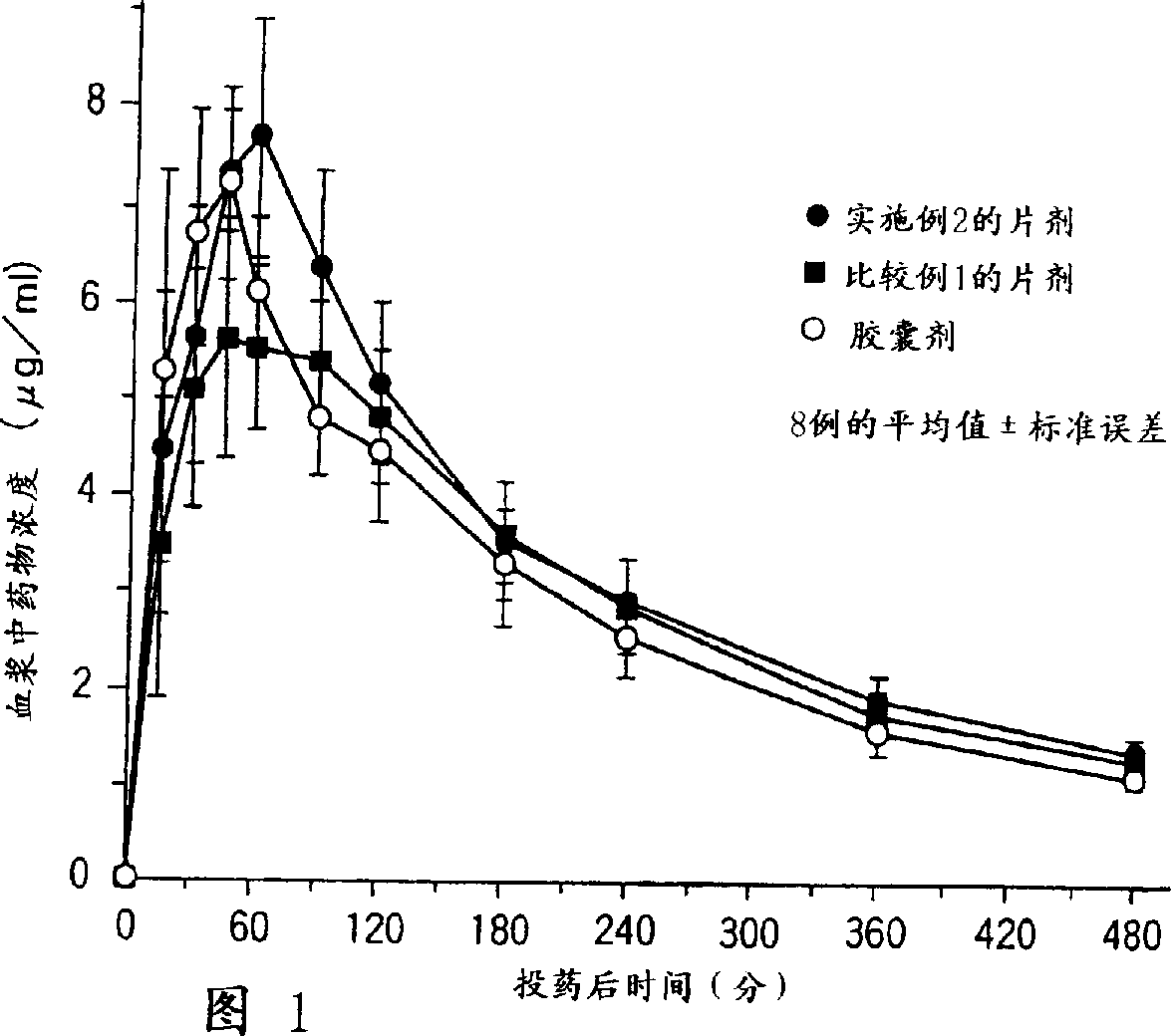
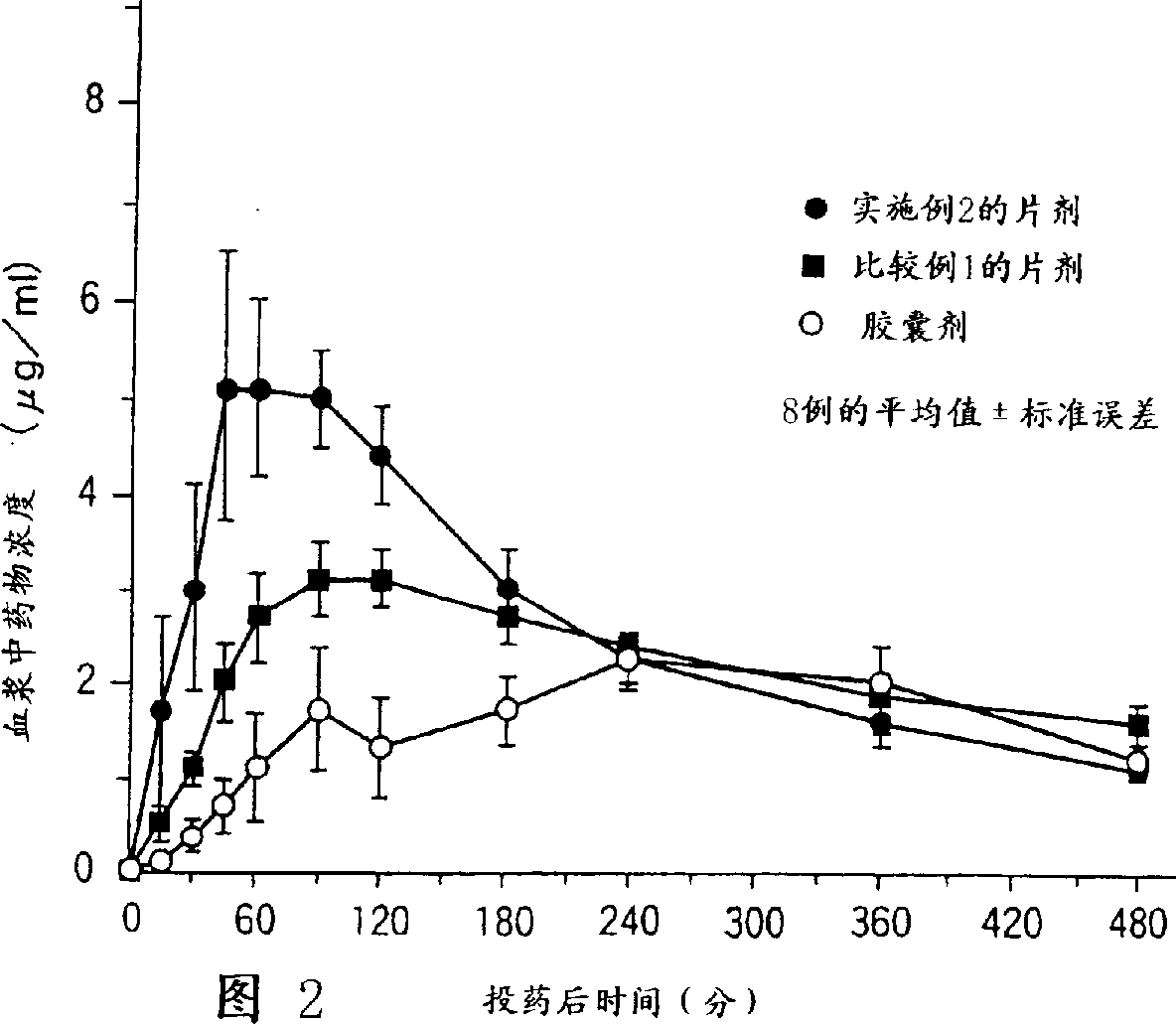
[0033]For the obtained coated tablet, the influence of food on the oral absorbability was evaluated in the same manner as described above. As a resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com