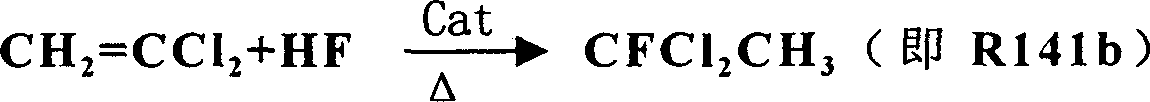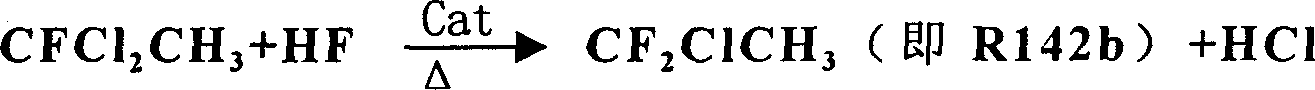Process for preparing 1,1,1-trifluoroethane and 1,1,1-difluorochloroethane
A technology of difluorochloroethane and trifluoroethane, which is applied in the field of preparation of halogenated hydrocarbons, can solve the problems of rising production costs, complicated recycling process, and large equipment investment, and achieves improved utilization rate, simple process, and low equipment investment. little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
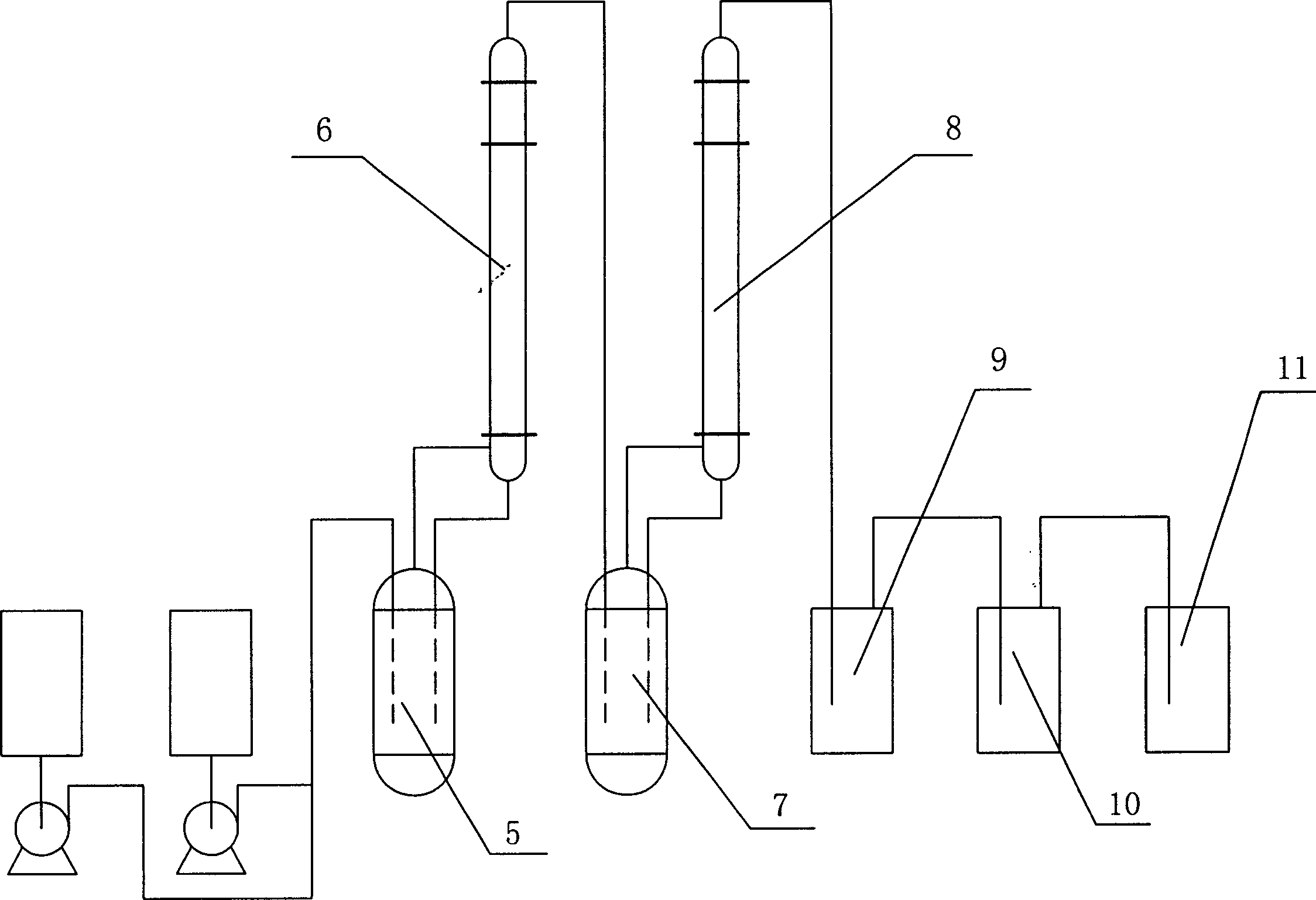
[0024] Embodiment 1: see attached figure 1 As shown, first put 200g SnCl 4 Catalyst and 300g anhydrous HF are pressed in the 1000ml steel first reactor 5 that has reflux tower 6, and be warmed up to 55 ℃; Put 20g SbCl 5 Catalyst and 400g of R142b primer are pressed into the 1000ml steel second reactor 7 with reflux tower 8, and the temperature is raised to 50°C. Then, begin to anhydrous hydrogen fluoride with the speed of 56g / h, vinylidene chloride is sent to react in the first reactor 5 with the speed of 97g / h, after the pressure in the first reactor 5 reaches 1.2Mpa, from The top of the reflux tower of the first reactor 5 introduces the reaction materials into the second reactor 7 for further reaction. After the pressure of the second reactor 7 reached 1.0Mpa, the reaction product was drawn from the top of the reflux tower of the second reactor 7, and the reaction product was collected in the gas cabinet 11 after washing with water in the washing tower 9 and alkali washing...
Embodiment 2-4
[0026]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com