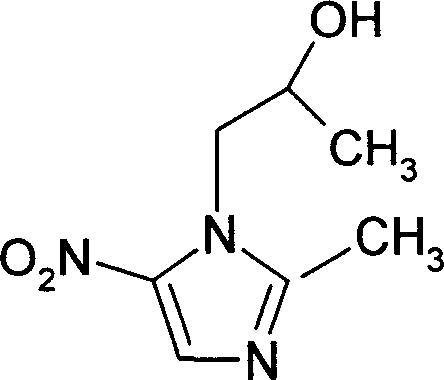
Improved method of preparing secnidazole
A kind of technology of other butnidazole and nitro group is applied in the field of preparing other butnidazole-2-methyl-5-nitro-1H-imidazole), can solve problems such as low yield, and achieve the effect of less consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
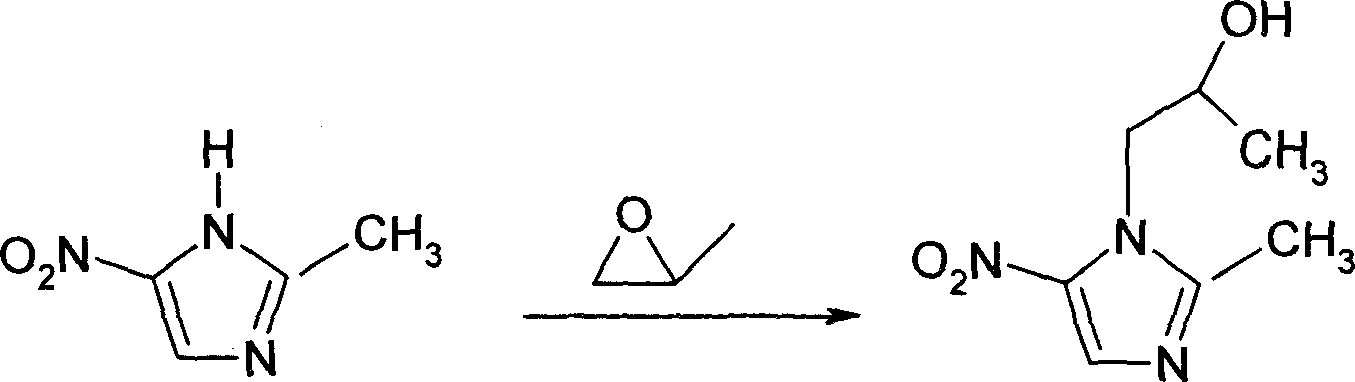
[0030] 2-Dimethyl-5-nitro-1H-imidazole-1-ethanol (i.e. another butinidazole, also known as 1-(2-hydroxypropyl)-2-methyl-5-nitro-1H-imidazole ) preparation method
[0031] In a 5-liter four-necked flask with a cooling device and a dropping funnel with a jacket, add 1600ml of anhydrous ethyl acetate, cool to 0-5 degrees Celsius under stirring, and slowly add 400g (3.0mol) of anhydrous chlorinated Aluminum, keep the temperature between 0-5 degrees Celsius. To the resulting clear solution was added 200 g (1.5748 mol) of 2-methyl-5-nitro-imidazole. Observe for a thick slurry. Keep the temperature at 0-5 degrees Celsius, add 160 g (2.75 mol) of propylene oxide dropwise, and control the dropping rate at about 1 hour. The reactant was continuously stirred for 3 hours at a temperature of 0-5 degrees Celsius, then poured into diluted hydrochloric acid, and stirred for 15 minutes. The reaction solution was filtered to remove unreacted 2-methyl-5-nitro-imidazole. Dry at -80°C for 24 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com