Viral clearance process
A technology of immunoglobulin and cut-off value, which is applied to the preparation method of peptides, chemical instruments and methods, disinfection, etc., and can solve problems that do not involve the removal of contaminating hepatitis virus particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Virus-free RhoGAM(R) was produced by the following ultrafiltration method.
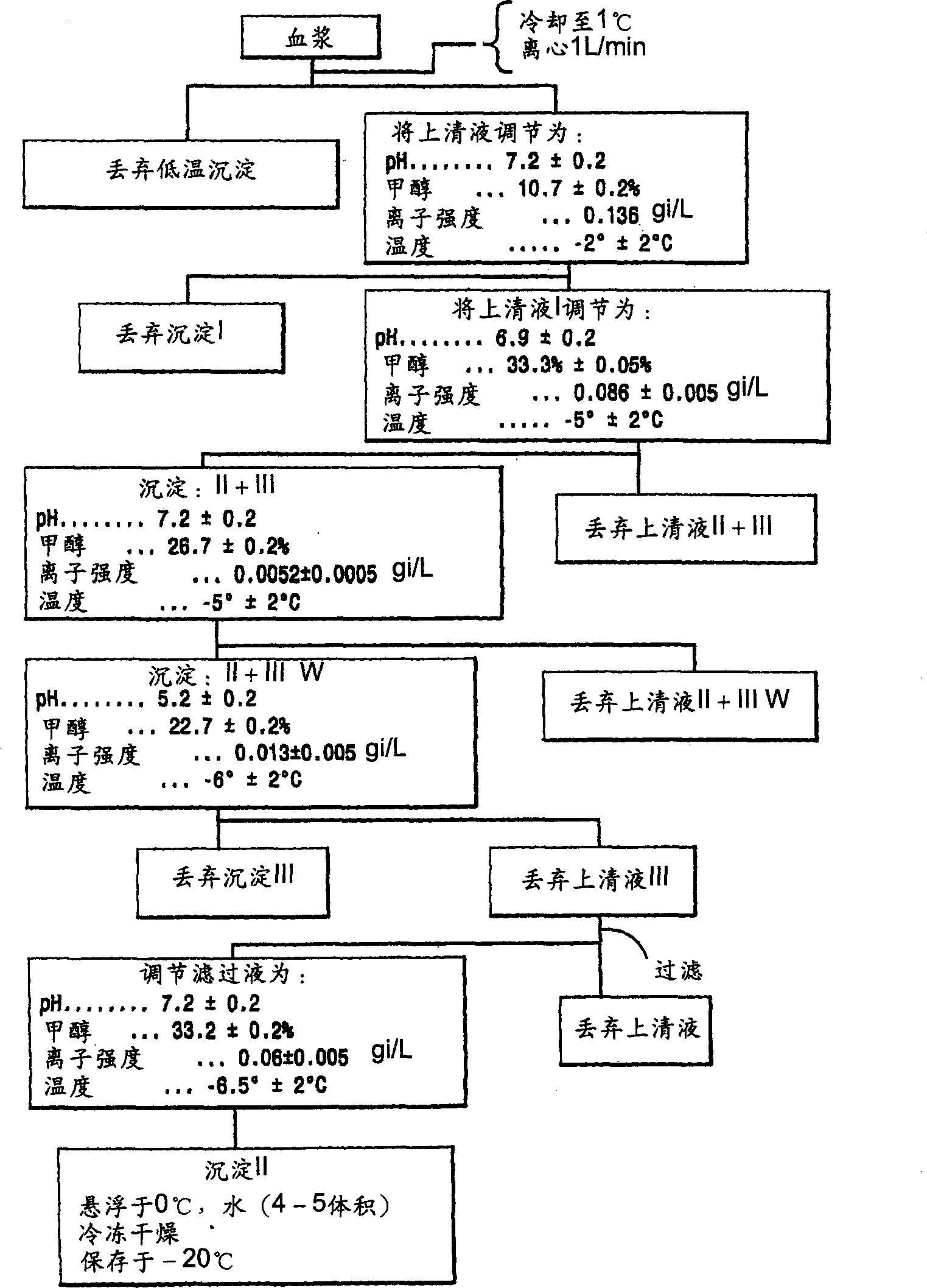
[0118] Rho(D) immunoglobulin 3.250 kg was purified to "Pellet II" by the modified Cohn purification method (see above), which was resuspended in 9.750 L of water for injection (WFI) U.S.P. and cooled to 5°C. The mixture was vortexed (no foaming) for 4 hours and stored at 4°C until use. The weight of the precipitate II resuspension was 3.250 kg.
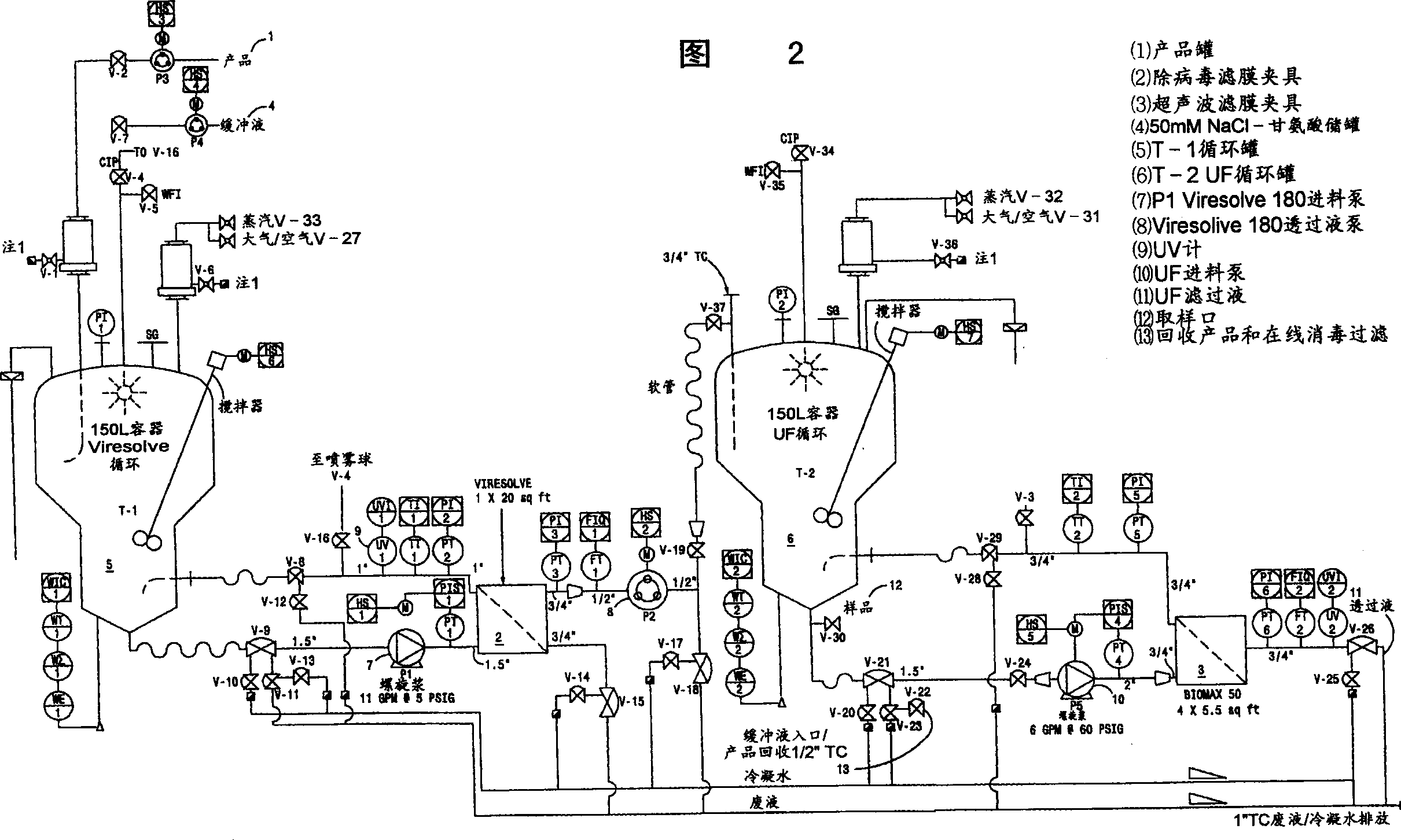
[0119] After SIP, a Viresolve-180R module (Millipore Corporation) (10 stacks) suitable for approximately 13.0 L of Precipitate II resuspension was installed. The Pellicon CIP / SIP module was replaced with 2 Biomax-50 filter frames. The Viresolve-180R module is chlorine sterilized and washed. Rinse the Biomax-50 filter frame with WFI U.S.P. Benzalkonium chloride (Roccal) was assayed on the last permeated rinse sample; benzalkonium chloride concentration was 6 ppm. Diffusion tests were performed on Biomax-50 filter frames; the release rate was calcu...
Embodiment 1A
[0153] The 150 mM NaCl-glycine buffer used in Example 1 was prepared as follows:
[0154] Calculate the amount of buffer to prepare:
[0155] [Resuspension volume (L)×10L]×2+60=buffer preparation volume
[0156] [13L×10L]×2+60=prepare 320L buffer
[0157] Determine and measure the amount of raw material needed, and add to a depyrogenated container.
[0158] raw material
required concentration
× per batch
= required amount
NaCl
8.87g / L
302L
2,838.4g
15.01g / L
320L
4,803.2g
Polysorbate 80
0.02g / L
320L
6.4g
[0159] raw material
required concentration
× per batch
= required amount
1.0N NaOH
0.125ml / L
320L
40ml
[0160] The mixture was diluted with Water for Injection, U.S.P., and the resulting mixture was mixed for 60 minutes. Measure the pH; the requirement is 6.3-6.5. The actual pH is 6.46. If not, add ...
Embodiment 2
[0164] In Example 2, virus-free RhoGAM® was produced by ultrafiltration as previously described with the following modifications:
[0165] Purify 6.802 kg of Rho(D) immunoglobulin to "precipitate II slurry" by the modified Cohn purification method, resuspend in 20.406L water for injection (WFI) U.S.P., and cool to 4°C. The mixture was vortexed (no foaming) for 4 hours and stored at 4°C until use.
[0166] After SIP, a Viresolve-180R module (Millipore Corporation) (20 stacks) for 27.208 L of Precipitation II resuspension was installed. The Pellicon CIP / SIP module was replaced with 2 Biomax-50 filter frames. The Viresolve-180R module is chlorine sterilized and washed. Rinse the Biomax-50 filter frame with WFI U.S.P. Benzalkonium chloride (Roccal) was assayed on the last permeated rinse sample; benzalkonium chloride concentration was 8 ppm. Diffusion testing was performed on a Biomax-50 filter frame; the release rate was calculated as previously described; the total release ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com