Process for preparing 1,2,4-benzothiadiaxine-1,1-dioxide compound
A technology of dioxo compounds and benzothiadiazine, which is applied in the field of preparation of 1,2,4-benzothiadiazine-1,1-dioxo compounds, can solve the problem of low yield in the cyclization process, Problems such as low yield and long number of processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
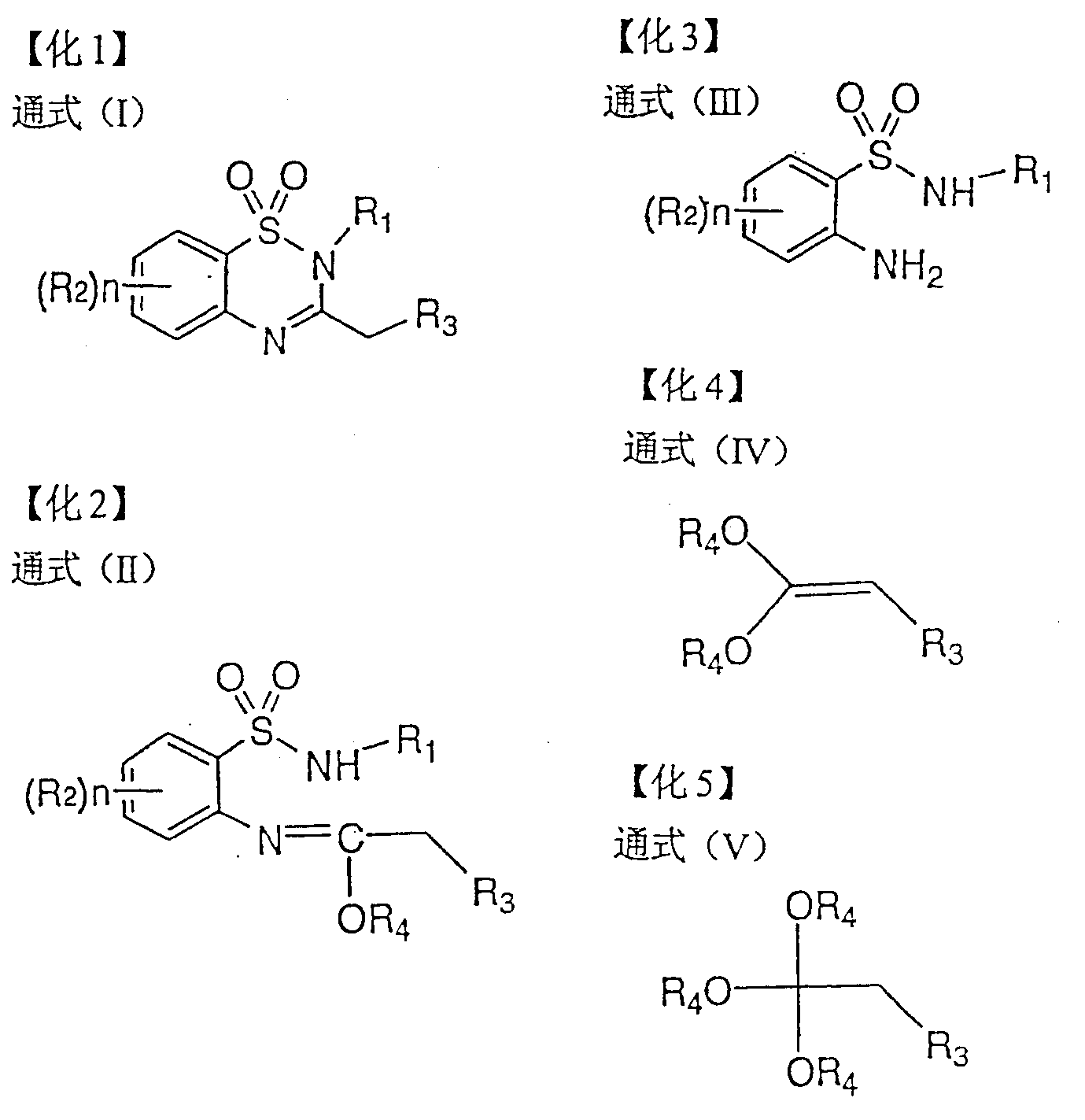
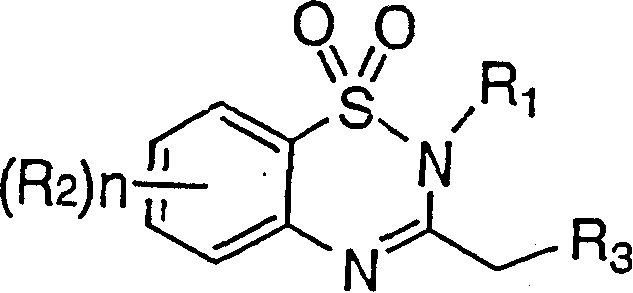
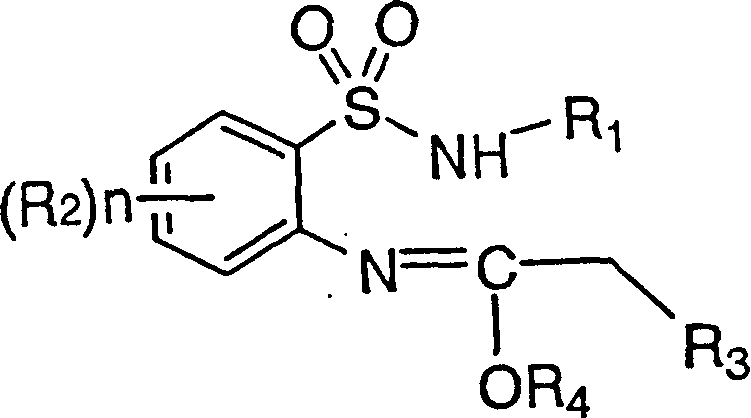
[0117] In the preparation method of the present invention, the process of synthesizing the compound represented by the general formula (I) itself in the synthesis stage of the compound represented by the general formula (III) can also be used without separating intermediates in the way, or similarly using simple extraction, Preparation by concentrating the reaction solution, etc.
[0118] Specifically, after the reaction of o-nitrobenzenesulfonyl chlorides and amines, the starting material for the synthesis of the compound represented by the general formula (III), the nitro group can be reduced without separation and the resulting general formula (III) can be separated. Represented aniline compound, then make it react with the compound represented by general formula (IV) or (V), without separating the intermediate represented by general formula (II), carry out continuous reaction once synthetic general by - pot method (same reaction tank) The compound represented by the formul...
Embodiment 1
[0132] Synthesis of Exemplified Compound (8)
[0133] Exemplary compound (8) was synthesized as follows.
[0134] 【Chemical 32】
[0135]
[0136] To 10.0g (23.6mmol) compound (A-1), 5.32g (28.3mmol) 3,3-diethoxy ethyl acrylate (A-0) and 0.045g (0.24mmol) p-toluenesulfonic acid monohydrate 20ml of toluene was added, heated to reflux for 30 minutes. After adding 0.226 g (2.4 mmol) of sodium tert-butoxide, it was heated to reflux for 5 hours. After cooling the reaction solution to room temperature, dilute hydrochloric acid was added for neutralization, and 30 ml of ethyl acetate was added. The organic solvent layer was washed with water, dried over sodium sulfate, and the solvent was distilled off under reduced pressure. Crystallization from methanol gave 11.2 g of the target exemplified compound (8). Yield 91.1%. Melting point: 80-81°C.
[0137] 1 H-NMR (300MHz, CDCl 3 )11.72(s, 1H), 778(d, 1H), 7.58(t, 1H), 7.19(t, 1H), 7.10(d, 1H), 4.61(s, 1H), 4.20(q, 2H), 2.27(...
Embodiment 2
[0138] Synthesis of Exemplified Compound (8)
[0139] Add 20ml of xylene to 8.49g (20mmol) of compound (A-1), 4.54g (24mmol) of 3,3-diethoxy ethyl acrylate (A-0) and 0.60g (6.52mmol) of acetic acid, and heat to reflux 30 minutes. After addition of 3.04 g (20 mmol) DBU, it was heated to reflux for 7 hours. After cooling to room temperature, dilute hydrochloric acid was added for neutralization, and 30ml of ethyl acetate was added. After washing with water, the organic solvent layer was dried with sodium sulfate. After the solvent was distilled off under reduced pressure, 8.78 g of the target exemplary compound (8) were obtained by crystallization from methanol. Yield 84.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com