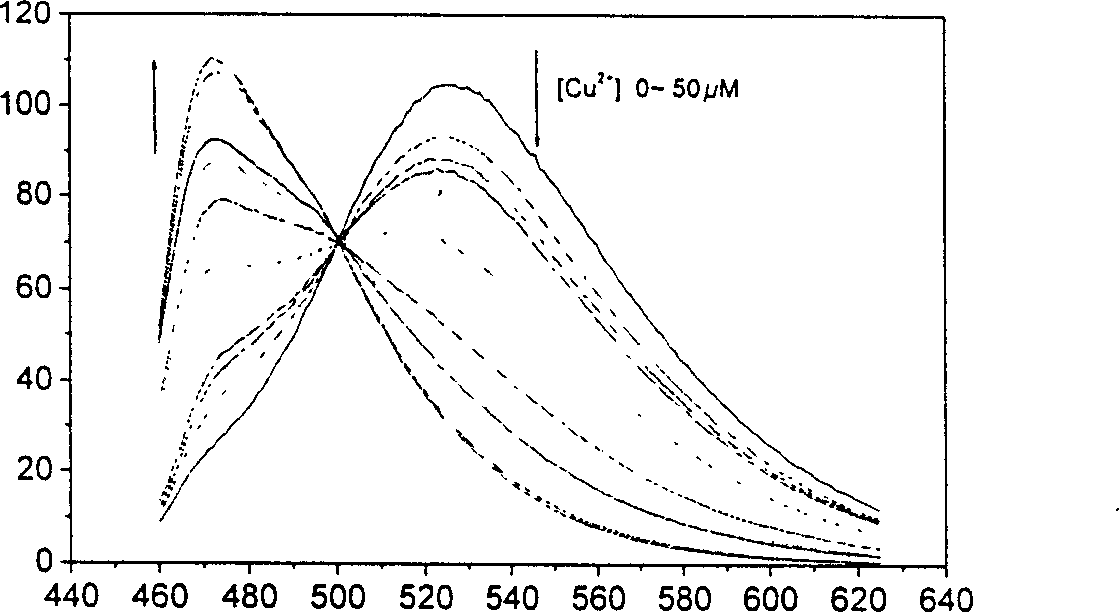
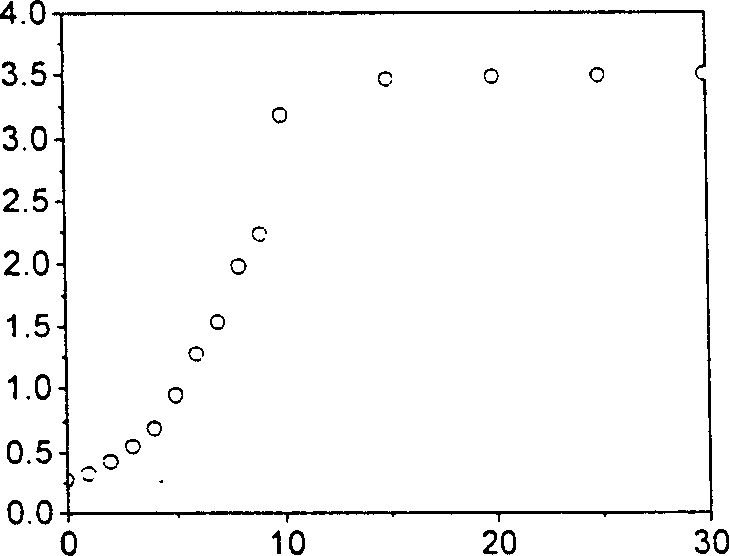
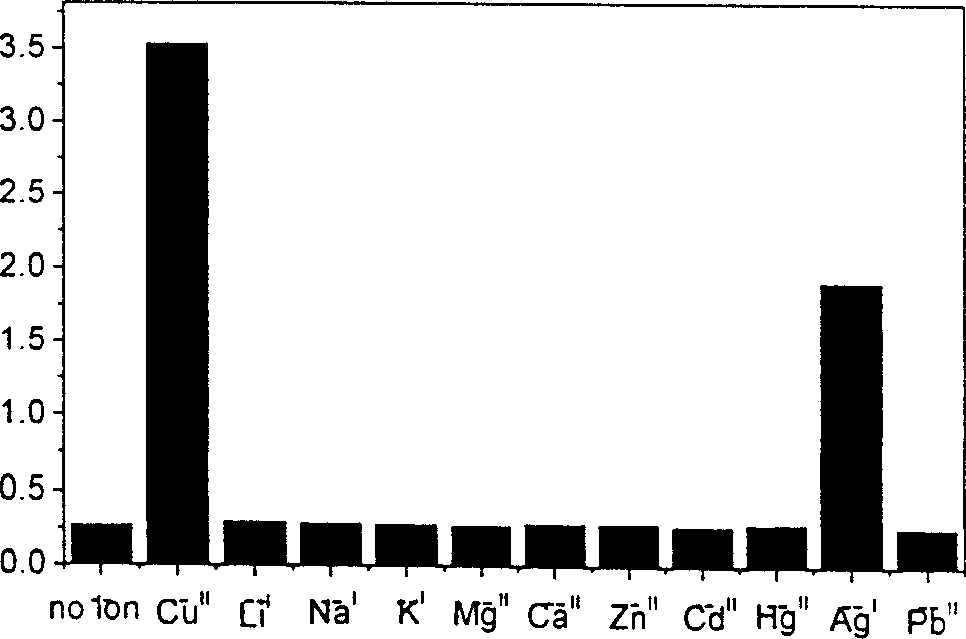
4,5-disubstituted-1,8-naphthoyl imide compounds and use thereof
A technology of naphthalene imide and compound, which is applied in the field of highly selective photosensitive identification and ratio detection, and achieves the effects of easy availability of raw materials, high derivatization and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027]Add 0.1g (0.31mmol) 4-bromo-5-nitro-1,8-naphthalene anhydride in a 25ml flask, add 10ml ethanol and heat to reflux, cool to 50°C after a while, slowly add 0.0227g butane Amine (0.31mmol) in 5ml ethanol solution, the originally cloudy solution suddenly became clear, and the color also deepened, turning red. Heating continued to reflux, and the TLC plate followed the reaction after 40 min. After distillation of ethanol under reduced pressure, silica gel column separation, CHCl 3 elute. Recrystallized from ethanol to finally obtain the product as white needle-like crystals, weighing 47 mg, with a yield of 40.2%. The melting point is 175.8-176.2°C. 1 H-NMR (CDCl 3 , 400MHz) 60.99(t, J=7.2Hz, 3H), 1.45(m, J=7.2Hz, 2H), 1.71(m, J=7.2Hz, 2H), 4.18(t, J=7.2Hz, 2H) , 7.93(d, J=8.0Hz, 1H), 8.21(d, J=8.0Hz, 1H), 8.51(d, J=8.0Hz, 1H), 8.71(d, J=8.0Hz, 1H). 13 C-NMR (CDCl 3 , 100MHz), 613.95, 20.49, 30.21, 40.92, 122.69, 123.72, 124.26, 125.99, 131.37, 132.49, 136....
Embodiment 2
[0029]
[0030] In a 25ml flask, add 100mg (0.266mmol) N-butyl 4-bromo-5-nitro-1,8-naphthalimide, add 10ml ethylene glycol monomethyl ether to dissolve, add N-phenyl - Ethylenediamine 0.15ml (1.05mmol), heating. After 4min the reaction was over. Poured into water, extracted with dichloromethane, and purified by chromatographic column to obtain a yellow solid with a yield of 87%. The melting point is 186.2-187.5°C. 1 H-NMR (CDCl 3 , 400MHz): 60.946-0.983(t, J=7.6Hz, 3H), 1.401-1.458(m, J=7.6Hz, 2H), 1.654-1.711(m, J=7.6Hz, 2H), 3.640(br, 4H), 4.111-4.149(t, J=7.6Hz, 2H), 6.714-6.834(br, 4H), 7.221-7.262(t, J=8Hz, 3H), 7.752-7.771(d, J=7.6Hz, 1H), 7.826 (s, N-H), 8,264-8.284 (d, J=8Hz, 1H), 8.425-8.446 (d, J=8Hz, 1H).
Embodiment 3
[0032]
[0033] Dissolve more than 120mg of the product in 10ml of ethylene glycol monomethyl ether, then add 1ml of N-phenylethylenediamine, and reflux until the reaction is complete. It was poured into water, extracted with dichloromethane, and purified by chromatographic column to obtain a yellow solid weighing 136 mg with a yield of 74%. The melting point is 203.5-204.1°C. 1 H-NMR (CDCl 3 , 400MHz) δ0.936-0.972(t, J=7.2Hz, 3H), 1.395-1.450(m, J=7.2Hz, 2H), 1.669-1.704(m, J=7.2Hz, 2H), 3.351-3.425 (dd, J=5.2Hz, 4H) 4.104-4.140(t, J=7.2Hz, 2H), 6.195(s, N-H), 6.538-6.559(d, J=8.4Hz, 1H), 6.672-6.692(d , J=8.4Hz, 4H), 6.766-6.803(t, J=7.6Hz, 2H), 7.191-7.230(d, J=7.6Hz, 4H), 8.265-8.286(d, J=8.4Hz, 2H) . 13 C-NMR (CDCl 3 , 400MHz): δ14.060, 20.671, 30.608, 40.028, 43.474, 44.036, 107.252, 113.590, 119.010, 129.797, 132.26, 133.615, 147.893, 152.205, 164.661. HRMS(EI)calcd for C 32 h 35 N 5 o 2 [M] 521.2791, found 521.2779.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com