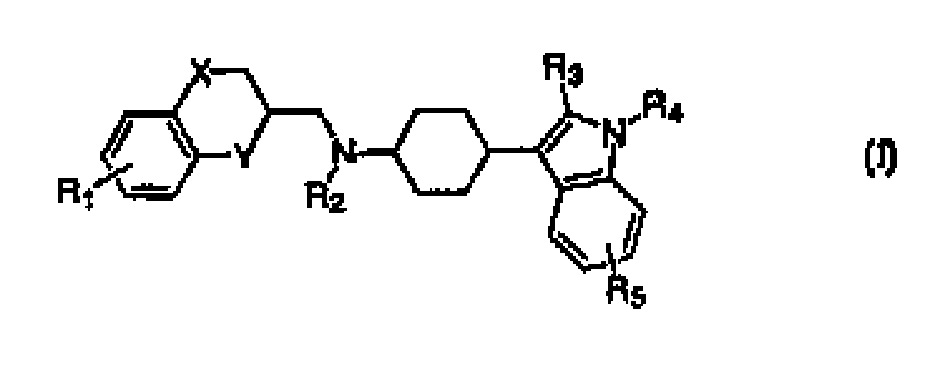
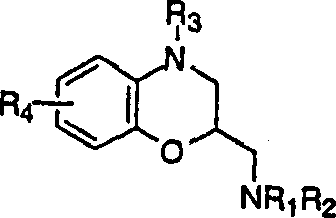
Indol-3-Yl-cyclohexyl amide derivatives for treatment of depression (5-HT1 receptor antagonists)
A cyclohexyl and indole technology, which is applied in the field of various indol 3-yl-cyclohexylamine derivatives for the treatment of such diseases, can solve the problems of weakened curative effect and inability to fully explain the therapeutic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 (3,4-dihydro-benzo[1,4]oxazin-2-ylmethyl)-[cis-4-(5-fluoro-1H-indol-3-yl)-ring Hexa]-amine and (3,4-dihydro-benzo[1,4]oxazin-2-ylmethyl)-[trans-4-(5-fluoro-1H-indol-3-yl) -
[0073] Cyclohexyl]-amine
[0074] Trifluoroacetic acid (5 ml) was added to cis / trans-(tert-butyl-3,4-dihydro-benzo[1,4]oxazine-4-carboxylate-2-methanol at room temperature yl)-4-[5-fluoro-1H-indol-3-yl)-cyclohexyl]-amine in dichloromethane (15ml). After stirring the reaction mixture for 2 hours at room temperature, the solvent was removed. A small amount of methanol was added to the residue, and the solution was adjusted to pH >9 with 2N sodium hydroxide solution. The aqueous portion was extracted with dichloromethane. The combined organic extracts were washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated. The product was purified by chromatography (ethyl acetate / methanol / ammonia: 99 / 1 / 0.5) to obtain 0.41 g (35%) of the cis isomer as a white solid: mp 65...
Embodiment 2
[0082] Example 2 (3,4-dihydro-benzo[1,4]oxazin-3-ylmethyl)-[cis-4-(5-fluoro-1H-indol-3-yl)-ring Hexa]-amine and (3,4-dihydro-benzo[1,4]oxazin-3-ylmethyl)-[trans-4-(5-fluoro-1H-indol-3-yl) -
[0083] Cyclohexyl]-amine
[0084] 4-(5-fluoro-1H-indol-3-yl)-cyclohexanone (0.578g, 2.5mmol), 3-aminomethyl-1,4-benzoxazine (0.411g, 2.5mmol) , sodium triacetate borohydride (0.78g, 3.5mmol) and acetic acid (0.14ml, 2.5mmol) in 1,2-dichloroethane (11ml) was stirred at room temperature for 5 hours. The reaction was quenched with 1N sodium hydroxide and extracted with dichloromethyl. The combined organic extracts were washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated. The product was purified by chromatography (ethyl acetate / methanol / ammonia: 99 / 1 / 0.5) to obtain 0.63 g (66%) of the cis isomer as an oil. The fumarate of the cis isomer was prepared in isopropanol: mp 208-209°C.
[0085] C 23 h 26 FN 3 O 0.5C 4 h 4 o 4 0.3H 2 Elemental analysis of O...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com