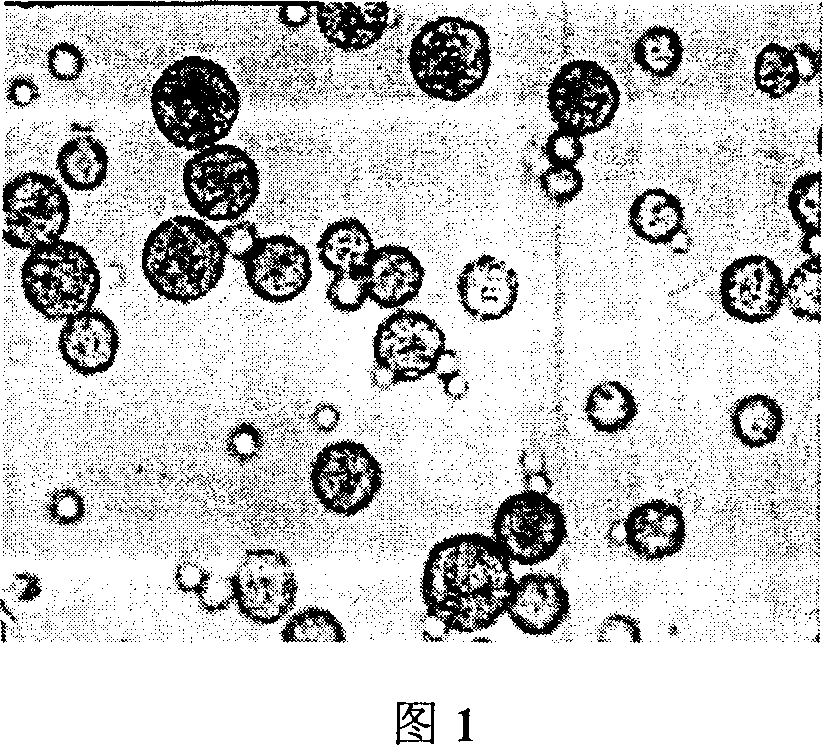
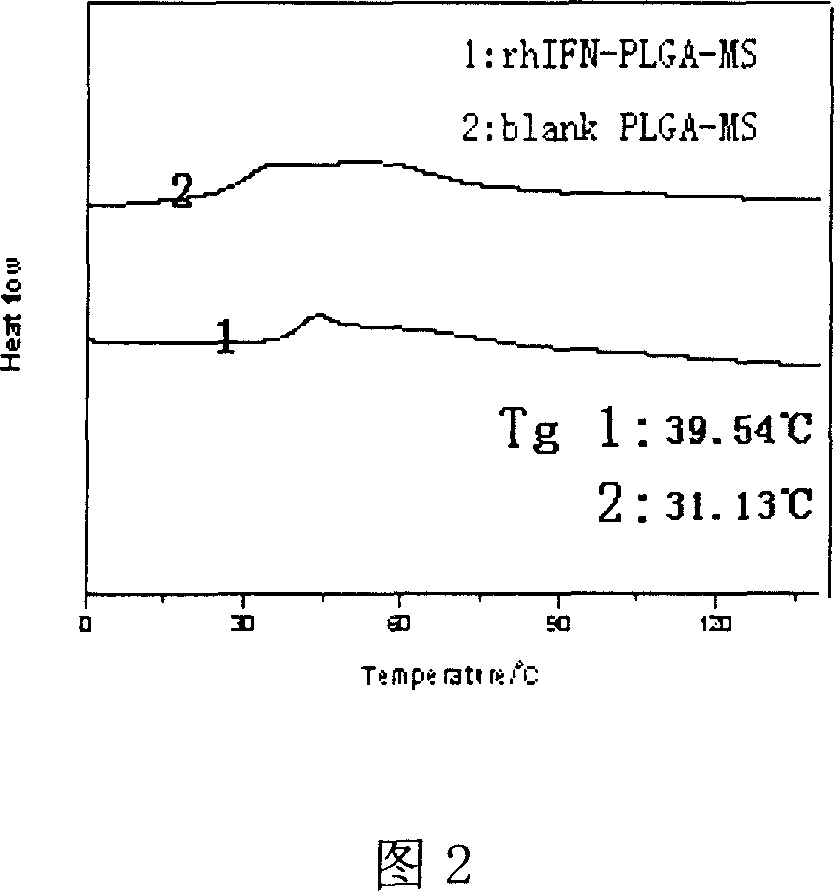
Method of preparing microsphere of ethoxyl copolymer PLGA in interferon poly acid
A technology of polylactic acid and interferon, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of short biological half-life, poor stability, hindering use, etc., and achieve smooth surface , Good fluidity, prolonging the effect of action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 Preparation of interferon poly(lactic-co-glycolic acid) (PLGA) microspheres
[0026] (1) Preparation of polylactic acid-glycolic acid copolymer (PLGA) solution
[0027] 400 mg of poly(lactic-co-glycolic acid) (PLGA) was dissolved in 2 ml of dichloromethane to form an organic phase (20% g / ml).
[0028] (2) Preparation of interferon PBS solution
[0029] Dissolve 40 mg of lyophilized recombinant human interferon-α for injection in 300 μl of 1% (g / ml) gelatin solution to form an internal aqueous phase;
[0030] (3) Preparation of colostrum
[0031] Inject the inner aqueous phase into the organic phase, stir and emulsify at 9000rpm for 1 minute to form colostrum (W / O);
[0032] (4) Preparation of double emulsion
[0033] Add colostrum to 300ml containing 1% (g / ml) PVA and 1% (g / ml) NaCl solution (outer water phase) under the situation of stirring at 2000rpm, emulsify for 10 minutes, emulsify into double emulsion (W / O / W);
[0034] (5) Formation of microsp...
Embodiment 2
[0039] Embodiment 2 Preparation of interferon poly(lactic-co-glycolic acid) (PLGA) microspheres
[0040] (1) Preparation of polylactic acid-glycolic acid copolymer (PLGA) solution
[0041] 600 mg of poly(lactic-co-glycolic acid) (PLGA) was dissolved in 2 ml of dichloromethane to form an organic phase (30% g / ml).
[0042] (2) Preparation of interferon PBS solution
[0043] Dissolve 60 mg of lyophilized recombinant human interferon-α for injection in 300 μl of 30% PEG400 solution to form an inner aqueous phase;
[0044] (3) Preparation of colostrum
[0045] Inject the inner aqueous phase into the organic phase, stir and emulsify for 3 minutes at 1000rpm to form colostrum (W / O);
[0046] (4) Preparation of double emulsion
[0047] Add colostrum to 200ml containing 3% (g / ml) PVA and 0.5% (g / ml) NaCl solution (outer water phase) under the situation of stirring at 1600rpm, emulsify for 20 minutes, emulsify into double emulsion (W / O / W);
[0048] (5) Formation of microspheres ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com