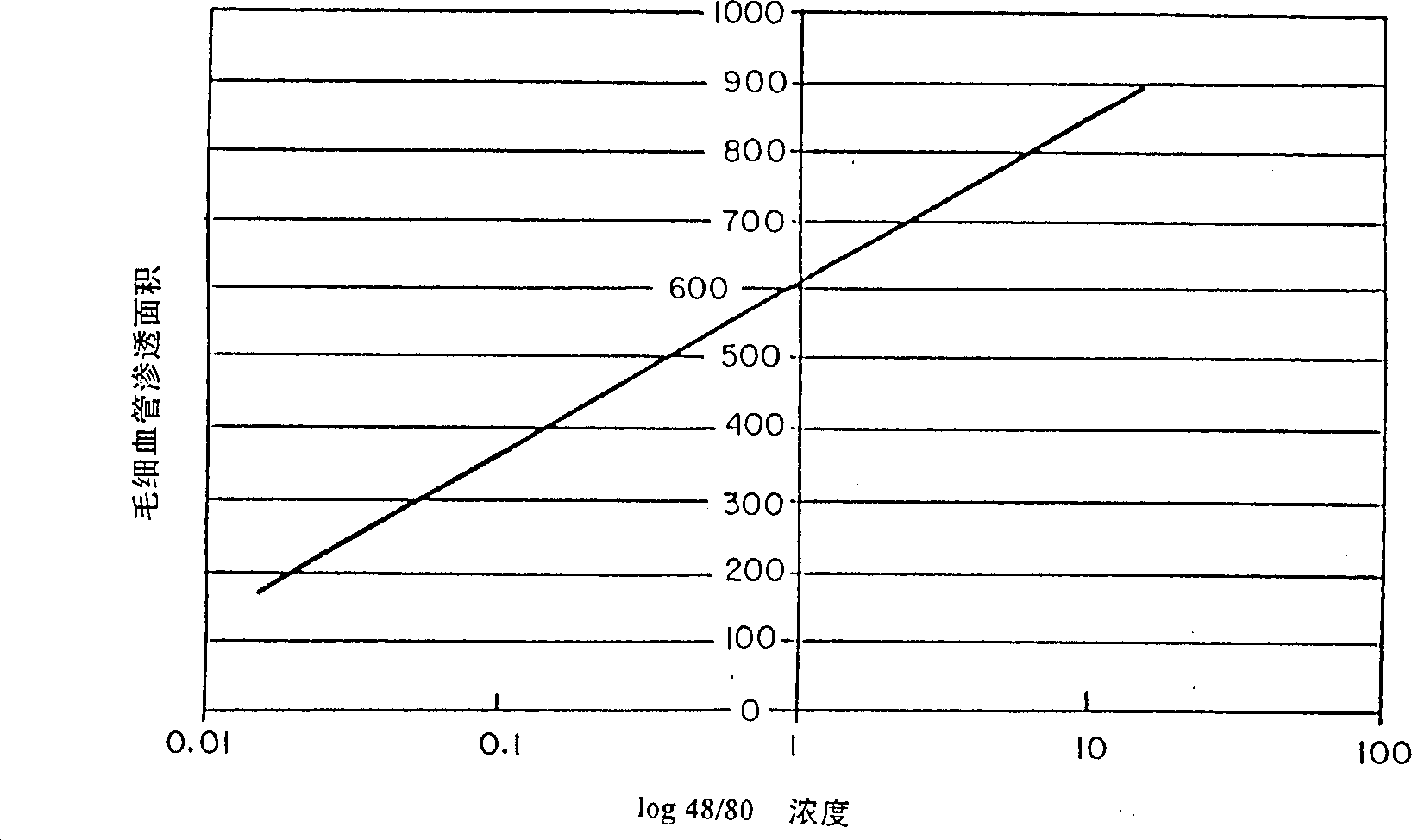
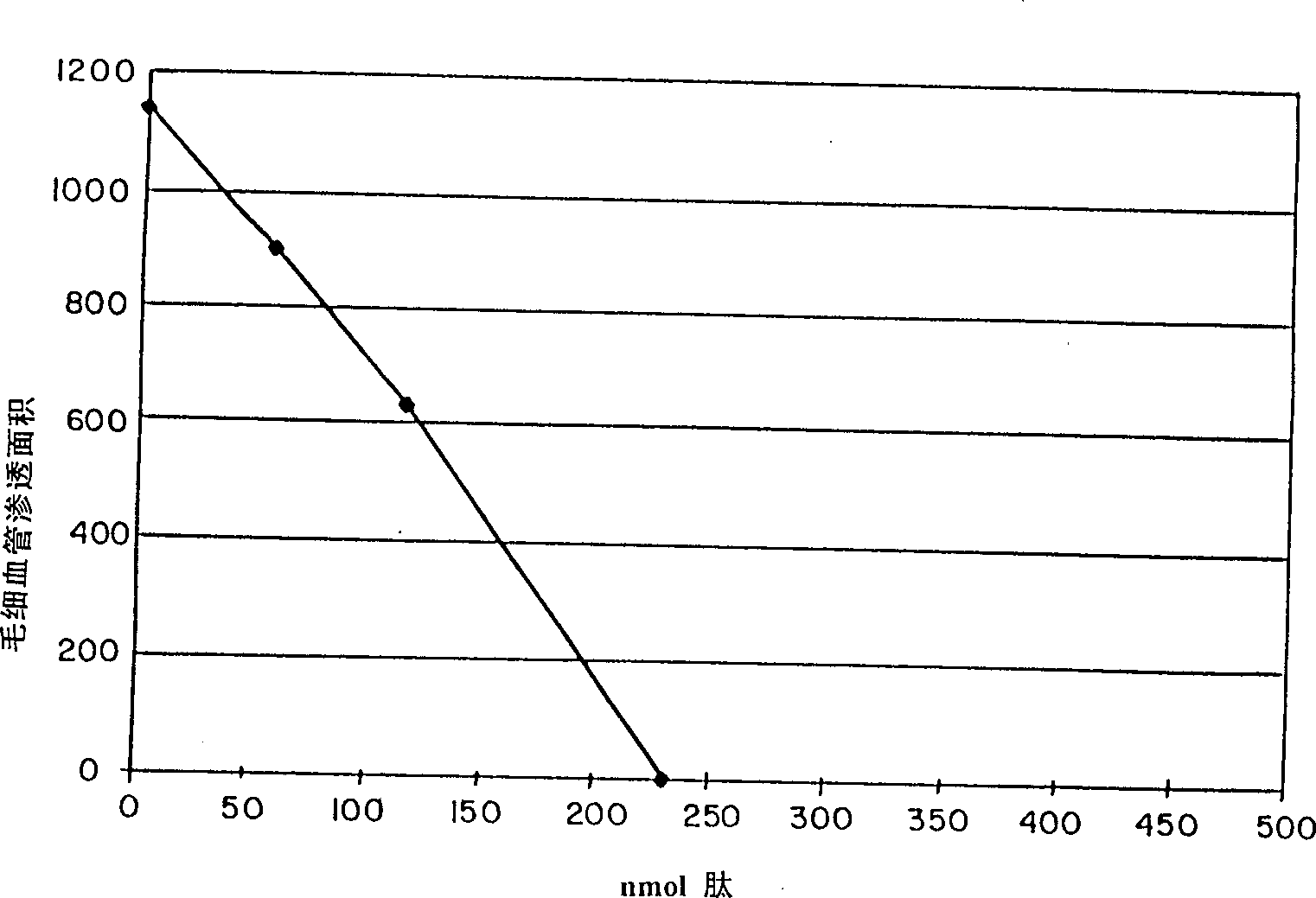
Small peptides and methods for treatment of asthma and inflammation
A skin inflammation, selected technology, applied in the direction of peptides, tripeptide components, tetrapeptide components, etc., can solve the problem of inflammation without any effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The compositions of the invention may conveniently be presented in unit dosage form and may be prepared by any of the methods well known in the art of pharmacy. Methods of preparation thereof generally include the step of bringing into association the active ingredient of the invention with the carrier which constitutes one or more accessory ingredients.
[0054] Compositions of the invention suitable for administration by inhalation may be used, for example, as aerosols or solutions for inhalation. An example of a typical aerosol composition consists of the desired amount of microcrystalline peptide and oleic acid suspended in a mixture of trichlorofluoromethane and dichlorodifluoromethane. An example of a typical solution consists of the desired amount of peptide, benzalkonium chloride, and Sulfuric acid (to adjust pH) composition.
[0055] Compositions of the invention suitable for oral administration may also be presented as discrete units such as capsules, cachet...
Embodiment 1
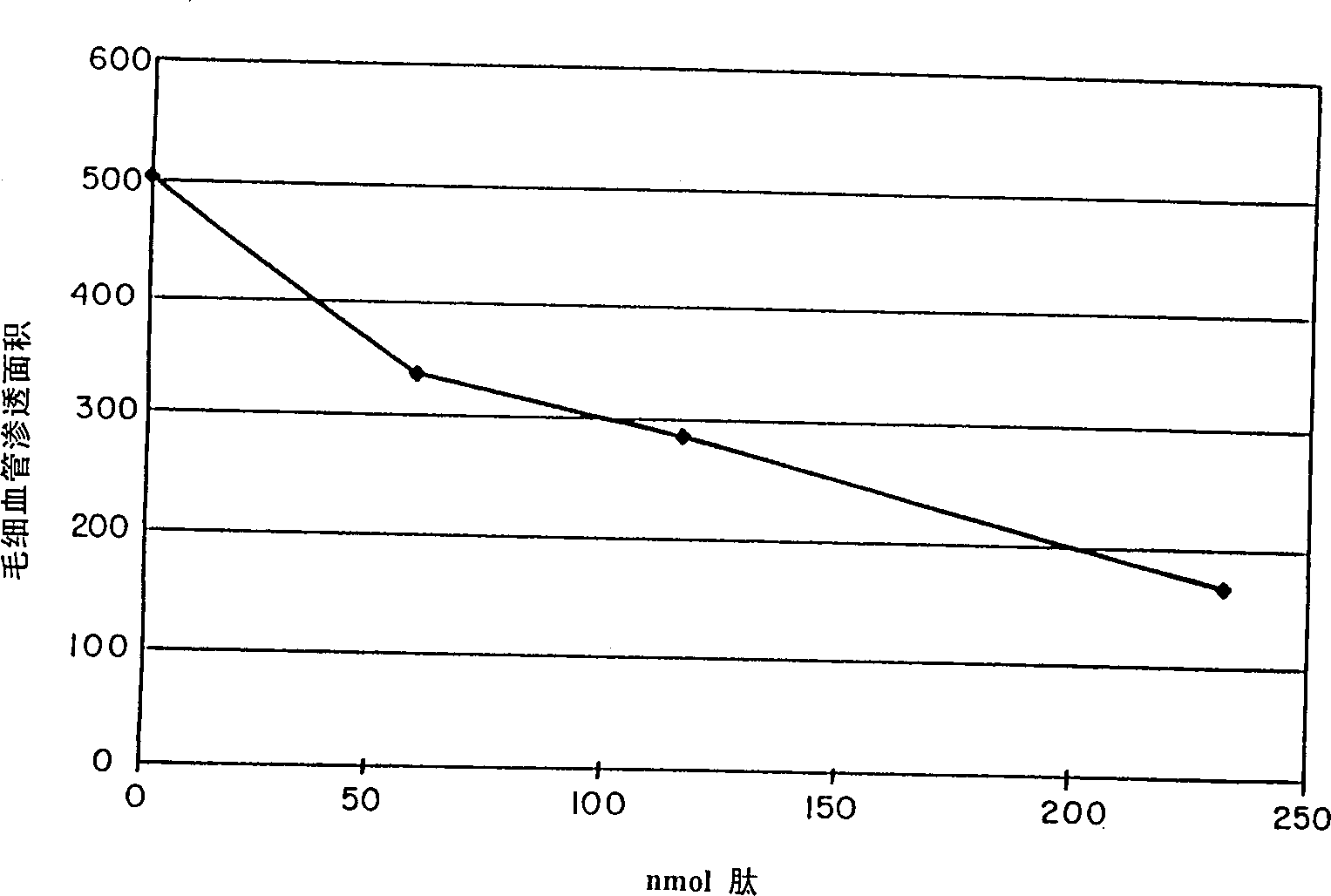
[0070] A dose response curve of the standard compound f-Met-Leu-Phe was prepared using the selected 0.15 μg dose of compound 48 / 80. f-Met-Leu-Phe was tested at doses ranging from 0 to about 230 nM, and the results are attached figure 2 shown. The results clearly show that degranulation induced by compound 48 / 80 is inhibited.
Embodiment 2-11
[0072] The potency of several f-Met-Leu peptides to inhibit induced degranulation was determined using 100 nanomolar test peptide and 0.15 μg compound 48 / 80 in a rat skin model. For each experiment, an internal zero-peptide-dose 48 / 80 control was included for each rat, and % inhibition was expressed as a percentage relative to this control (0% inhibition). The percentage of mast cell degranulation produced by 48 / 80 was also determined. The results are listed in the table below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com