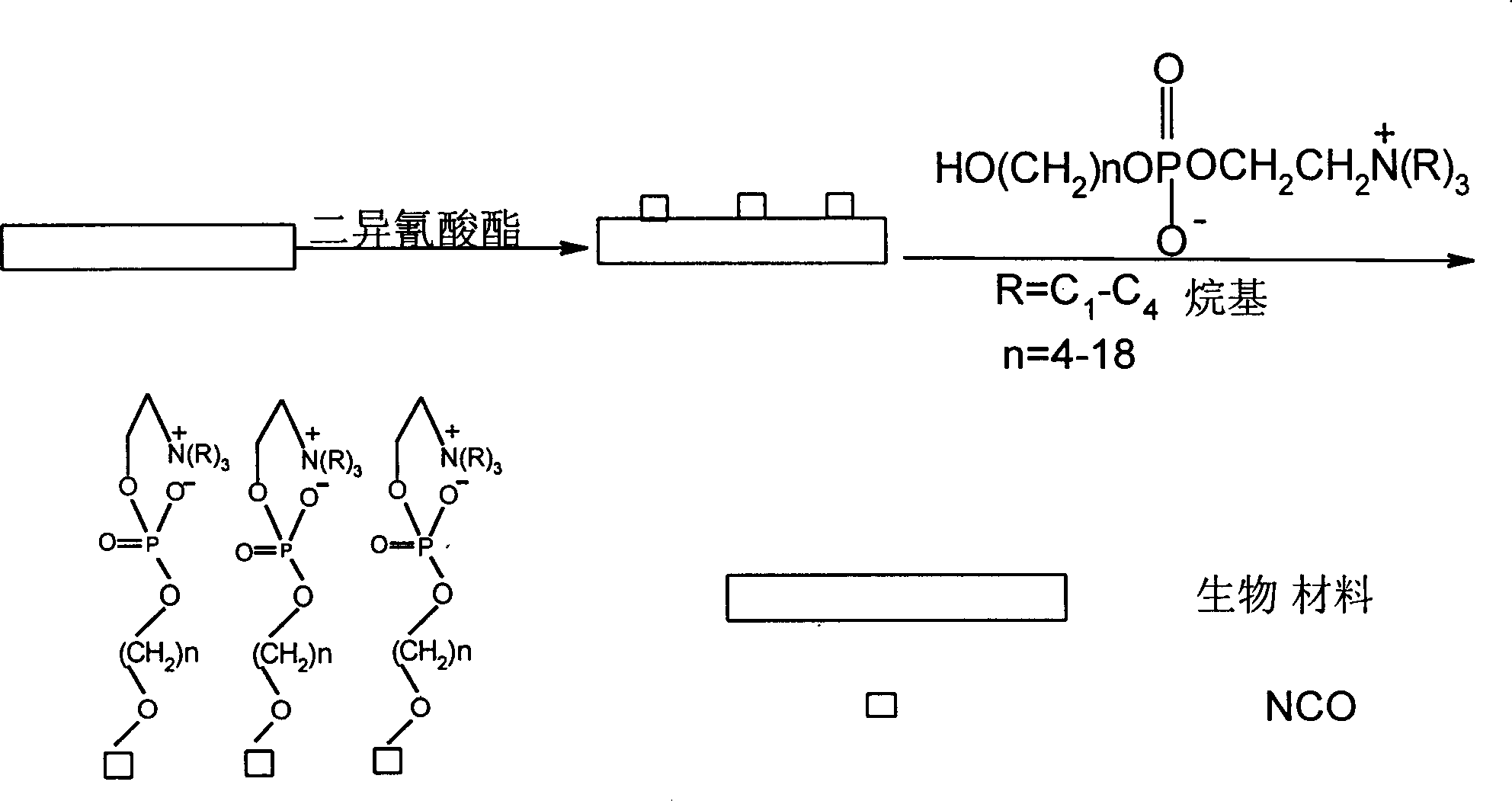
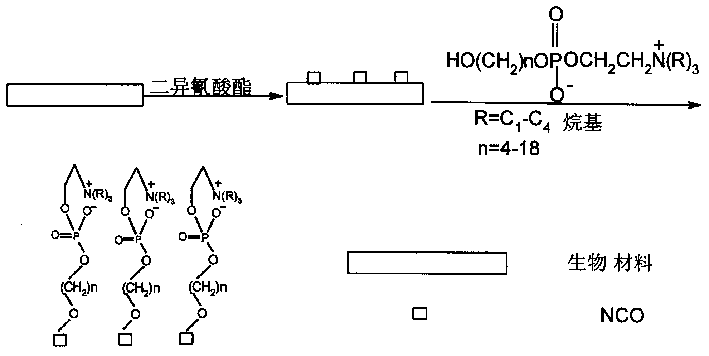
Phosphonic choline containing hydroxy, its preparing process and process for preparing biological material containing it
A technology of phosphorylcholine and biomaterials, which is applied in the field of non-coagulant biomaterials and its preparation method, and can solve the problems of decreased mechanical properties, long synthetic routes, and low utilization of phosphorylcholine structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The preparation of 2-(4-hydroxy)butoxy-2-oxo-1,3,2-dioxaphospholane: a certain amount of 2-chloro-2-oxo-1,3,2 - Dioxaphospholane (COP) is dropped into 100-200ml tetrahydrofuran (THF) containing 1 mol equivalent of butanediol and 1 mol equivalent of triethylamine, and react at -5—-30°C for 2-10 hours . The crude product was obtained by filtering off the amine salt, and the crude product was purified to obtain a pure product.
[0016] Through elemental analysis, FT-IR spectroscopy and 1 The H NMR spectrum confirmed that the product had the expected molecular structure of 2-(4-hydroxy)butoxy-2-oxo-1,3,2-dioxaphospholane.
[0017] The preparation of 4-hydroxy-2-butylphosphorylcholine: quickly pour a little excess trimethylamine into a certain amount of 2-(4-hydroxy)butoxy-2-oxo-1,3,2-di Seal the steel cylinder of oxaphospholane and anhydrous acetonitrile, and react at 40-90°C for 24-72 hours to obtain a yellow viscous crude product, which is further purified to obtain a ...
Embodiment 2
[0020] Preparation of 2-(5-hydroxy)pentyloxy-2-oxo-1,3,2-dioxaphospholane: a certain amount of 2-chloro-2-oxo-1,3,2 - Dioxaphospholane (COP) is dropped into 100-200ml tetrahydrofuran (THF) containing 1 mol equivalent of pentanediol and 1 mol equivalent of triethylamine, and react at -5—-30°C for 2-10 Hour. The crude product was obtained by filtering off the amine salt, and the crude product was purified to obtain a pure product.
[0021] Through elemental analysis, FT-IR spectroscopy and 1 The H NMR spectrum confirmed that the product had the expected molecular structure of 2-(5-hydroxy)pentyloxy-2-oxo-1,3,2-dioxaphospholane.
[0022] Preparation of 5-hydroxy-2-pentylphosphorylcholine: quickly pour a little excess trimethylamine into a certain amount of 2-(5-hydroxy)pentyloxy-2-oxo-1,3,2-di Seal the steel cylinder of oxaphospholane and anhydrous acetonitrile, and react at 40-90°C for 24-72 hours to obtain a yellow viscous crude product, which is further purified to obtain a...
Embodiment 3
[0025] The preparation of 2-(6-hydroxy)hexyloxy-2-oxo-1,3,2-dioxaphospholane: a certain amount of 2-chloro-2-oxo-1,3,2 - Dioxaphospholane (COP) is dropped into 100-200ml tetrahydrofuran (THF) containing 1 mol equivalent of hexanediol and 1 mol equivalent of triethylamine, and react at -5—-30°C for 2-10 hours . The crude product was obtained by filtering off the amine salt, and the crude product was purified to obtain a pure product.
[0026] Through elemental analysis, FT-IR spectroscopy and 1 The H NMR spectrum proved that the product had the expected molecular structure of 2-(6-hydroxy)hexyloxy-2-oxo-1,3,2-dioxaphospholane.
[0027]The preparation of 6-hydroxy-2-hexylphosphorylcholine: quickly pour a little excess trimethylamine into a certain amount of 2-(6-hydroxy)hexyloxy-2-oxo-1,3,2-dioxo Seal the steel cylinder of phospholane and anhydrous acetonitrile, and react at 40-90°C for 24-72 hours to obtain a yellow viscous crude product, which is further purified to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com