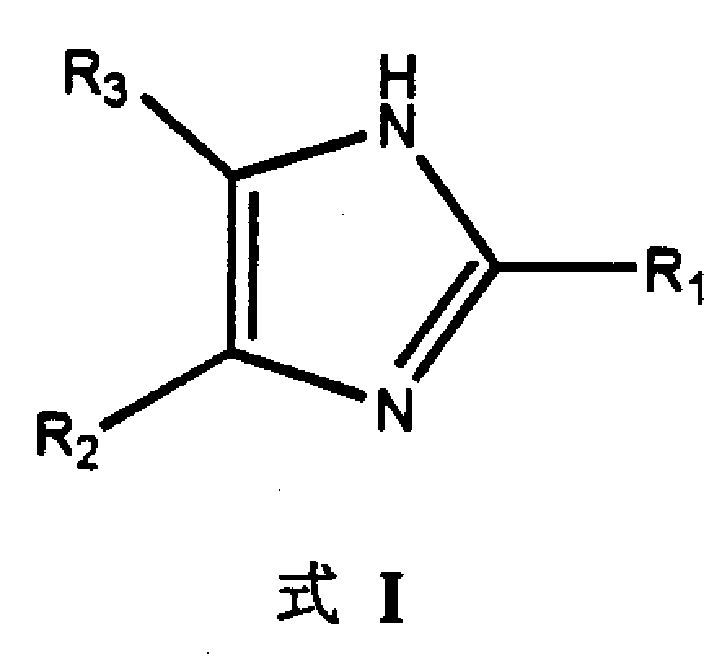
2,4,5-trisubstituted imidazole compounds and its preparing process and pharmaceutical use
A technology of compounds and substituents, applied in organic chemistry and other directions, can solve problems such as affecting functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 0.07g (0.3mmol) Pd(OAc) 2 , 0.37g (1.22mmol) PPh 3 Placed in 60ml of DMF, added 5g (27mmol) p-bromobenzaldehyde, 2.32g (27mmol) methyl acrylate, reacted at 100°C for 14h, separated by column chromatography to obtain a white solid product 1, weighing 4.80g, melting point 82.5°C, product The rate is 93.47%. 1 H-NMR (500MHz, CDCl 3 ): δ3.83(S, 3H), 6.56(d, 1H), 7.67(m, 2H), 7.72(d, 1H), 7.90(m, 2H), 10.04(S, 1H); MS(FAB) (m / z): 191 (base peak, M+H), 159 (M-OCH 3 ), 131 (M-COOCH 3 ), 105 (M-CH=CHCO 2 CH 3 ). Compound 1 Example 2: Synthesis of 1-(p-ethylene glycol formalyl phenyl)-trans-methyl acrylate (compound 2)
Embodiment 2
[0044] Dissolve 1.90g (10mmol) of 1 in 60ml of benzene, add 1.50g (24.2mmol) of ethylene glycol, install a water separator, reflux for 3h, cool to room temperature, add 20ml of ethyl acetate, and then use saturated NaHCO 3 , NaCl solution (each 2 × 20ml), washed with anhydrous Na 2 SO 4 After drying, the solvent was distilled off to obtain a light yellow solid, which was separated by column chromatography to obtain a white solid product 2, weighing 2.25 g, with a yield of 96.15%. 1 H-NMR (500MHz, CDCl 3 ): δ3.81(S, 3H), 4.09(m, 4H), 5.83(s, 1H), 6.46(d, 1H), 7.53(m, 4H), 7.70(d, 1H); MS(FAB) (m / z): 235(M+H), 203(M-OCH 3 ), 175 (M-COOCH 3 ), 149 (base peak, M-CH=CHCO 2 CH 3 ). Compound 2 Example 3: Synthesis of 1-(p-ethylene glycol formalyl phenyl)-trans-propenyl alcohol (compound 3)
Embodiment 3
[0045] At room temperature, add 30ml absolute anhydrous diethyl ether and 0.4gLiAlH 4 (10.54mmol) of 2.34g (10mmol) 30ml of absolute anhydrous ether solution was slowly dropped into the mixed suspension, and the drop was completed in about 20 minutes. Stir for another 60 minutes, add 10 ml of 1M NaOH aqueous solution, stir for 20 minutes, stand to separate layers, wash the ether layer with saturated NaCl solution, wash with anhydrous NaOH 2 SO 4 After drying, the solvent was distilled off to obtain a light yellow thick liquid, which was separated by column chromatography to obtain the white solid product 3, weighing 1.65 g, with a yield of 80.10%. 1 H-NMR (500MHz, CDCl 3 ): δ2.81(br, 1H), 4.09(m, 4H), 4.23(dd, 2H), 5.76(s, 1H), 6.32(dt, 1H), 6.55(d, 1H), 7.37(m, 4H); MS(FAB)(m / z): 207(M+H), 189(M-OH), 149(M-CH=CHCH 2 OH), 73 (base peak). Compound 3 Example 4: Synthesis of p-ethylene glycol formalyl phenyl-trans-allyl chloride (compound 4)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com