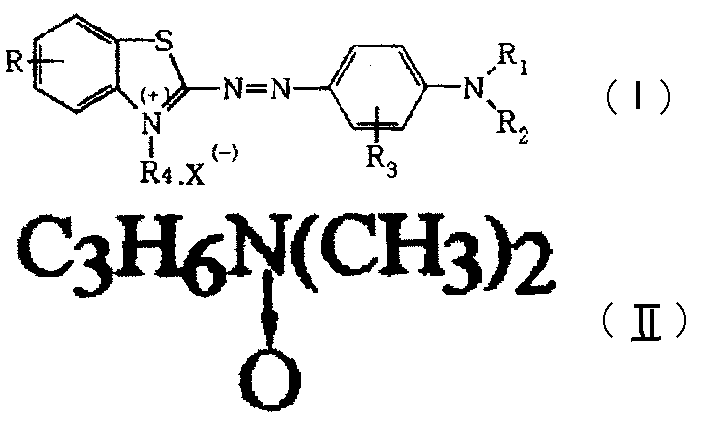Olive green cationic dye and its preparing method
A technology of cationic dyes and cationic precursors, which is applied in the field of olive green cationic dyes and its preparation, can solve the problems of poor reproducibility, level dyeing, troublesome operation, etc., and achieve the effects of easy operation, high dyeing rate and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 18.0 grams (0.1 moles) of 2-amino-6-methoxybenzothiazole and 260 grams of 30% sulfuric acid into the flask, stir to dissolve, add 0.4 grams of Swire oil after cooling, and 64 grams of Nitrosyl sulfuric acid composed of 93% sulfuric acid and 7.7 grams of sodium nitrite was slowly added, about 1 hour, stirred for 2 hours after the addition, diluted into 600 milliliters of ice water for later use. In another beaker, add 22.2 grams (0.1 moles) of N-ethyl-N-(N', N'-dimethylaminopropyl) aniline oxide and 25 grams of 30% sulfuric acid, 45 milliliters of water, stir to dissolve , add this coupling liquid to the above-mentioned diazo compound dilution below 5°C, stir for 2 hours to couple, after the end point is reached, slowly add about 340 grams of 30% liquid caustic soda for neutralization, so that the heterocyclic azo compound is precipitated, Filter and wash until neutral. Add this heterocyclic azo compound into a flask with 180 grams of chloroform and 2.4 grams of mag...
Embodiment 2
[0025] Add 19.4 grams (0.1 moles) of 2-amino-6-ethoxybenzothiazole, 76 grams of 98% sulfuric acid, and 160 grams of water into the flask, stir to dissolve, and dissolve 61 grams of 98% sulfuric acid at 0°C to 5°C Sulfuric acid and nitrosylsulfuric acid composed of 7.7 grams of dry sodium nitrite were added slowly for about 1.5 hours, stirred for 2 hours after the addition, and diluted into 600 milliliters of ice water for later use. In another beaker, add 25.6 grams (0.1 moles) of N-ethyl-N-(N', N', N'-trimethylaminopropyl) aniline chloride and 25 grams of 30% hydrochloric acid, 40 milliliters of water to make Stir to dissolve, add this coupling solution to the above-mentioned diazonium dilute solution below 5°C, stir for 2 hours to couple, after the end point is reached, slowly add about 330 grams of 30% liquid caustic soda for neutralization, and then salt out to make the heterocyclic Azo compounds were precipitated, filtered and dried. Add this heterocyclic dichloro dye to...
Embodiment 3
[0027] Except that the coupling component in embodiment 1 is replaced by 20.6 grams (0.1 moles) of N-ethyl-N-(N', N'-dimethylaminopropyl) aniline, all the other steps, formulas and reaction parameters are the same Embodiment 1, obtain 35 grams of former dyestuffs, make commodity dyestuffs through commercialization again.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com