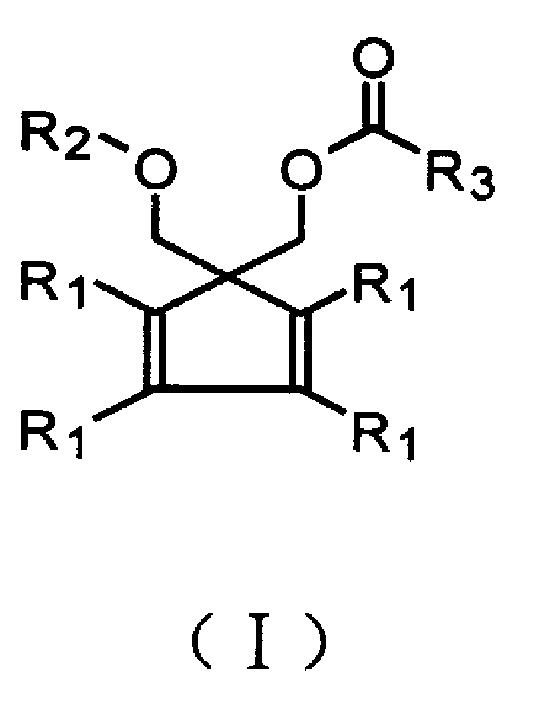
Š�-acyloxy group substituted ether compound utilized for preparing olefinic polymerization catalyst
A technology for substituting ethers and acyloxy groups, which is applied in the application field of preparing olefin polymerization catalysts, can solve the problems of undisclosed preparation methods of γ-acyloxy substituted ether compounds, and achieve improved stereospecificity, improved balance activity, and excellent performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0012] 1-methoxymethyl-1-acetoxymethyl-1,3-cyclopentadiene,
[0013] 1-methoxymethyl-1-propionyloxymethyl-1,3-cyclopentadiene,
[0014] 1-methoxymethyl-1-benzoyloxymethyl-1,3-cyclopentadiene,
[0015] 1-methoxymethyl-1-acetoxymethyl-2,3,4,5-tetramethyl-1,3-cyclopentadiene,
[0016] 1-methoxymethyl-1-propionyloxymethyl-2,3,4,5-tetramethyl-1,3-cyclopentadiene,
[0017] 1-methoxymethyl-1-benzoyloxymethyl-2,3,4,5-tetramethyl-1,3-cyclopentadiene,
[0018] 1-methoxymethyl-1-acetoxymethylindene,
[0019] 1-methoxymethyl-1-propionyloxymethylindene,
[0020] 1-methoxymethyl-1-benzoyloxymethylindene,
[0021] 1-methoxymethyl-1-acetoxymethyl-2,3-dimethylindene,
[0022] 1-methoxymethyl-1-propionyloxymethyl-2,3-dimethylindene,
[0023] 1-methoxymethyl-1-benzoyloxymethyl-2,3-dimethylindene,
[0024] 9-methoxymethyl-9-acetoxymethylfluorene,
[0025] 9-methoxymethyl-9-propionyloxymethylfluorene,
[0026] 9-methoxymethyl-9-benzoyloxymethylfluorene,
[0027] 9-methoxymethyl-9-acetox...
Embodiment 29
[0060] The IR characteristic absorption of the product is: 3320.71cm -1 (OH), 1098.00cm -1 (C-O). The preparation of embodiment 29-methoxymethyl-9-acetoxymethylfluorene
[0061] In an anhydrous and nitrogen atmosphere, dissolve 12g of 9-methoxymethyl-9-hydroxymethylfluorene in 100ml of benzene, slowly add 10g of acetic anhydride and 1 drop of concentrated sulfuric acid dropwise under stirring, and stir for 2 hours after the addition.
[0062] Add 2% sodium hydroxide aqueous solution and stir for 10 minutes, and the oily layer is washed with water once more, and then dried with anhydrous calcium chloride. After the solvent was evaporated, it was crystallized with absolute ethanol to finally obtain 12.8 g of the product with a yield of 91%.
[0063] With TMS as internal standard, in CDCl 3 In, the product's 1 The H-NMR spectrum is as follows:
[0064] 7.75ppm bimodal 2H aromatics
[0065] 7.61ppm bimodal 2H aromatics
[0066] 7.41ppm Trimodal 2H aromatics
[00...
Embodiment 3
[0072] The IR characteristic absorption of the product is: 1735.82cm -1 (C=O), 1100.19cm -1 (C-O). Preparation of Example 39-methoxymethyl-9-propionyloxymethylfluorene
[0073] In an anhydrous and nitrogen atmosphere, sequentially add 100ml of dichloromethane, 12g of 9-methoxymethyl-9-hydroxymethylfluorene and 7.6g of triethylamine, and stir to mix completely. Under cooling with ice water, 7 g of propionyl chloride was slowly added dropwise, and stirred for 1 hour after the addition was completed.
[0074] 100ml of ice water was added under stirring, and the oil layer was washed with 5% hydrochloric acid and sodium bicarbonate solution, and then dried with anhydrous calcium chloride. After the solvent was evaporated, it was crystallized with absolute ethanol to finally obtain 13.2 g of the product with a yield of 89%.
[0075] With TMS as internal standard, in CDCl 3 In, the product's 1 The H-NMR spectrum is as follows:
[0076] 7.74ppm bimodal 2H aromatics
[0077]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com