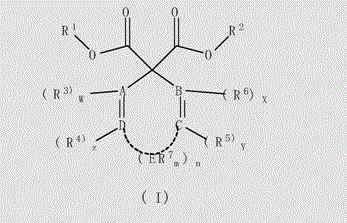
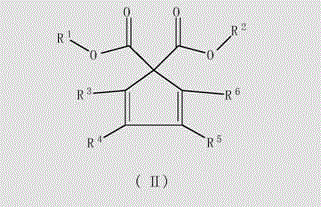
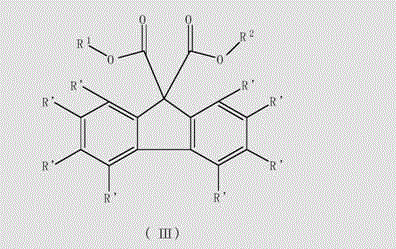
Unsaturated cyclosubstituted diacid ester compound suitable for preparing olefin polymerization catalyst
A technology of compounds and diacids, applied in the field of unsaturated ring-substituted diacids, which can solve the unsatisfactory balance of activity/isotacticity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Synthesis of fluorene-9-methyl formate-9-ethyl formate
[0053] Step A: Add 18g of sodium hydride, 50g of fluorene, and 150mL of toluene to a 1000mL three-necked flask in sequence under nitrogen protection, turn on mechanical stirring, heat up to 125°C under reflux, and keep the reaction for 4h; cool down to 90°C, and slowly add 146.1 g diethyl carbonate, drop it within 1.5 hours, and continue to react for 3 hours after the drop; cool to 20°C, slowly add a mixture of 60g concentrated hydrochloric acid and 75g water, and control the temperature not to exceed 40°C; filter and separate the organic phase, washed with water until neutral, and the organic phase was rotary evaporated to obtain a reddish-brown liquid; the liquid obtained by rotary evaporation was refluxed overnight with 157.4g of acetic acid and 63g of 10% hydrochloric acid; the mixture was lowered to 20°C and separated; the organic phase was rotary evaporated Then add 30% NaOH solution to adjust ...
Embodiment 2
[0057] Example 2 Synthesis of fluorene-9,9-diethyl carboxylate
[0058] (1.6M, 15mmol) n-butyllithium / hexane solution was added dropwise at -78°C to 20mL tetrahydrofuran solution containing 16mmol diisopropylamine, the above solution was stirred at -78°C for 45 minutes, and After stirring at 0°C for 20 minutes, the temperature was lowered to -78°C. A 20 mL tetrahydrofuran solution containing 7.0 mmol fluorene was added dropwise to the above stirring solution within 30 minutes at -78°C, and 33 mmol of ethyl chloroformate was added to the above mixture. The reaction system was warmed up to room temperature and stirred at room temperature for 3 hours. The above reaction mixture was poured into 100 mL of water, extracted with ether (three extractions, 50 mL of ether each time), the organic phase was dried with magnesium sulfate, and the crude product obtained after concentration was recrystallized with petroleum ether to obtain the product at 100-101°C.
[0059] Diethyl fl...
Embodiment 3
[0060] Example 3 Synthesis of fluorene-9,9-dimethyl-dicarboxylate
[0061] The synthesis steps are the same as in Example 2, except that ethyl chloroformate is replaced by methyl chloroformate.
[0062] Dimethyl fluorene-9,9-dicarboxylate 1 H-NMR (CDCl 3 )δ (ppm): 3.759 (s, 6H, methyl hydrogen), 7.359-7.392 (t, 2H, aromatic ring hydrogen), 7.443-7.475 (t, 2H, aromatic ring hydrogen), 7.720-7.735 (d, 2H , aromatic ring hydrogen), 7.799-7.7814 (d, 2H, aromatic ring hydrogen).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com