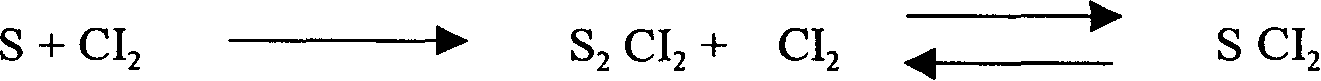Method for preparing sulfur dichloride
A technology of sulfur dichloride and disulfide dichloride, applied in chemical instruments and methods, chemical/physical processes, sulfur and halogen compounds, etc., can solve problems such as harm to the health of operators, environmental pollution, and inconvenience in implementation , to achieve the effect of facilitating popularization and application, no environmental pollution, and easy implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] In the preparation method of sulfur dichloride described in the present invention, the molar ratio of chlorine gas to sulfur is 2.05:1. Add sulfur and iron trichloride catalyst corresponding to 0.15% by weight of sulfur dichloride in the reaction kettle, then feed chlorine gas, control the reaction temperature to be 90-100°C, and control the reaction pressure to gauge pressure 0.1-0.15Mpa. Get disulfur dichloride;
[0018] Cool disulfur dichloride to 2-8°C, and feed chlorine gas to the set ventilation rate to produce sulfur dichloride product;
[0019] Add sulfur dichloride ethyl phosphite stabilizer of 0.2% by weight of sulfur dichloride after resting, obtain the finished product of sulfur dichloride with good stability, the purity reaches more than 98%, the storage period can reach 3 months, and the purity is not less than 95%. Easy to store and use.
[0020] Under the completely identical situation of other conditions, only the different stabilizers used for the pr...
Embodiment 2
[0023] In the preparation method of sulfur dichloride described in the present invention, the molar ratio of chlorine gas to sulfur is 2.06:1. Add sulfur and its equivalent iron trichloride catalyst of 0.12% by weight of sulfur dichloride in the reaction kettle, then feed chlorine gas, control the reaction temperature to be 80-90°C, and the reaction pressure to be controlled at a gauge pressure of 0.15-0.18Mpa. Get disulfur dichloride;
[0024] Cool disulfur dichloride to -6~-1°C, and then pass chlorine gas into it to produce sulfur dichloride product;
[0025] After standing still, 0.4% by weight of sulfur dichloride is added as a butyl phosphite stabilizer to obtain a finished product of sulfur dichloride.
Embodiment 3
[0027] In the preparation method of sulfur dichloride described in the present invention, the molar ratio of chlorine gas to sulfur is 2.03:1. Add sulfur and zinc chloride catalyst equivalent to 0.16% by weight of sulfur dichloride in the reaction kettle, then feed chlorine gas, control the reaction temperature to 110-120°C, and control the reaction pressure to a gauge pressure of 0.1-0.12Mpa to obtain Disulfur dichloride;
[0028] Cool disulfur dichloride to -8~-4°C, and then pass chlorine gas into it to produce sulfur dichloride product;
[0029] After resting, 0.5% by weight of sulfur dichloride is added as a dimethyl hydrogen phosphite stabilizer to obtain a finished product of sulfur dichloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com