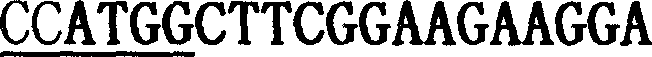Methods for the production of multimeric proteins, and related compositions
A technology of protein complexes and multimers, which is applied in the field of multimeric protein complexes and can solve problems such as difficulties in the preparation of multimeric proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0311] Isolation of thioredoxin and NADPH thioredoxin reductase genes
[0312] The Arabidopsis silique cDNA library CD4-12 was obtained from the Arabidopsis Biological Resource Center (ABRC, http: / / aims.cps.msu.edu) Arabidopsis collection and used as a template to isolate the mouse ear Thioredoxin h (Trxh) and thioredoxin reductase genes from mustard. In order to isolate the Trxh gene, the following primers were synthesized: GVR833: 5'TA 3' (SEQ ID NO: 1)
[0313] Bold shows the sequence identical to the 5' end of the Trxh gene published by Rivera-Madrid et al. (1993) Plant Physiol 102:327-328. Underlined are NcoI restriction sites to facilitate cloning. GVR834: 5'GA 3' (SEQ ID NO: 2)
[0314] Bold shows the sequence complementary to the 3' end of the Trxh gene published by Rivera-Madrid et al. (1993) Plant Physiol 102:327-328. Underlined is the HindII restriction site to facilitate cloning.
[0315] Polymerase chain reaction (PCR) was performed using GVR833 and GVR8...
Embodiment 2
[0320] Construction of plant expression vectors
[0321] Expression vectors were constructed to allow overexpression of thioredoxin and NADPH thioredoxin reductase in seeds in a seed-specific manner. The vector is constructed to allow overexpression at its natural subcellular location and accumulation on oil bodies.
[0322] Construction of Plant Transformation Vector pSBS2520
[0323]The Arabidopsis thaliana thioredoxin h gene described in Example 1 was placed under the control of the phaseolin promoter and phaseolin terminator derived from Phaseolus vulgaris (Slighton et al. (1983) Proc. Natl Acad Sc USA 80:1897-1901; Sengupta-Gopalan et al. (1985) PNAS USA 82:3320-3324)). Gene splicing by overlap extension technique (Horton et al. (1989) 15:61-68) fused the phaseolin promoter and Trxh gene. Standard molecular biology laboratory techniques (see, e.g., Sambrook et al. (1990) Molecular Cloning, 2nd edition, Cold Spring Harbor Press) were used to provide the phaseolin promot...
Embodiment 3
[0340] Polyacrylamide Gel Electrophoresis and Western Blotting of Transgenic Seed Extracts
[0341] Arabidopsis Thioredoxin, Thioredoxin Reductase and Oleosin
[0342] source of antibodies
[0343] The Arabidopsis thaliana thioredoxin and thioredoxin reductase genes were cloned in-frame into the bacterial expression vector pRSETB (Invitrogen) to allow expression of the Arabidopsis thaliana thioredoxin and thioredoxin reductase proteins. Express. These proteins were purified using standard procedures (see, eg, Invitrogen Protocols) and used to raise antibodies in rabbits by standard biochemical techniques (see, eg, Current Protocols in Molecular Biology, John Wiley & Sons, N.Y. (1989)). The Arabidopsis oleosin gene was cloned in-frame in the bacterial expression vector pRSETB (Invitrogen) to allow overexpression of the Arabidopsis oleosin. The protein is purified using standard procedures (see, eg, Invitrogen Protocols) and used to prepare mouse monoclonal antibodies by stan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com