Process for preparing lactic acid oligomer
A technology of lactic acid oligomers and mixtures, applied in the field of preparation of lactic acid oligomers, to achieve the effects of improving basic metabolism, lowering blood sugar, and suppressing excessive appetite
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
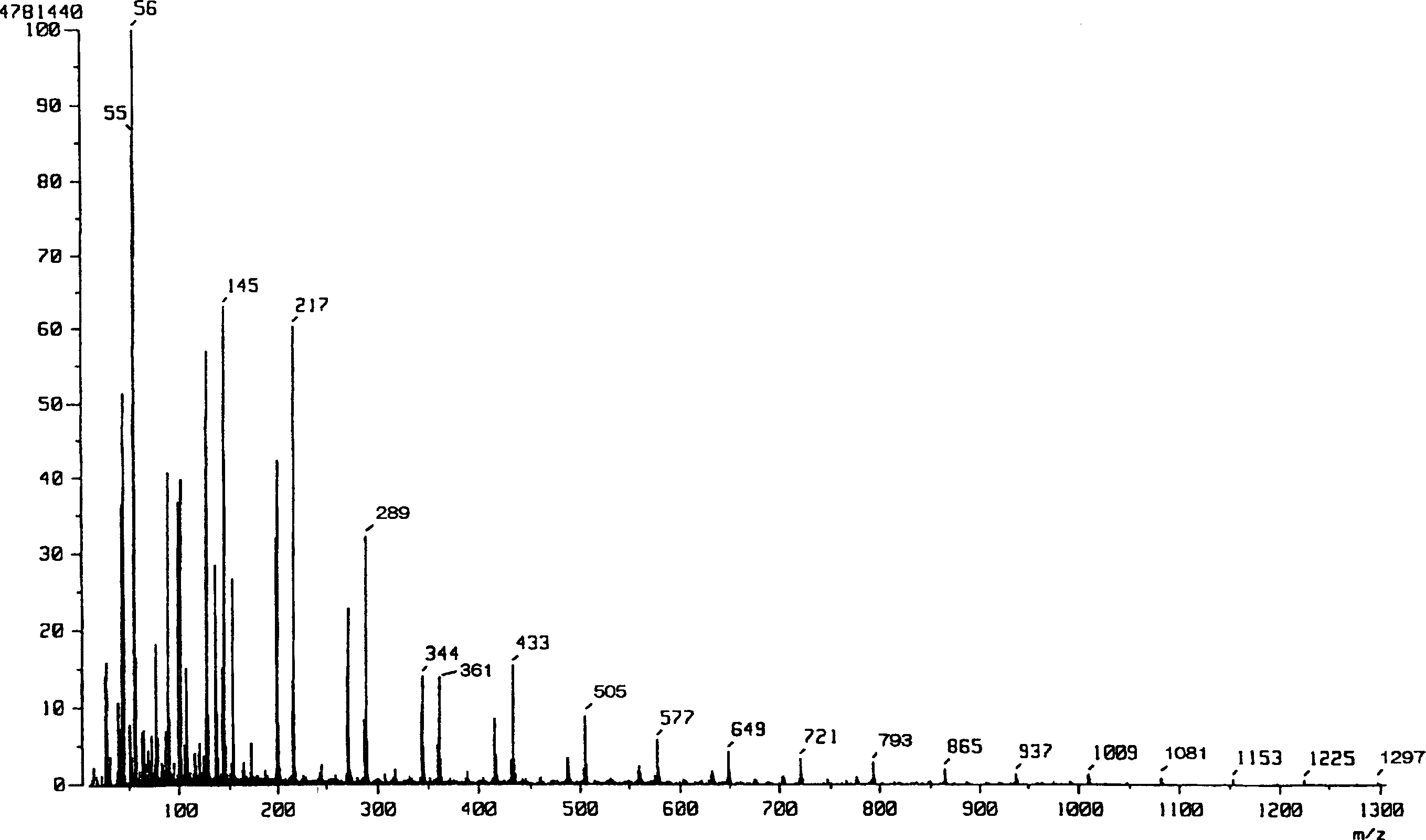
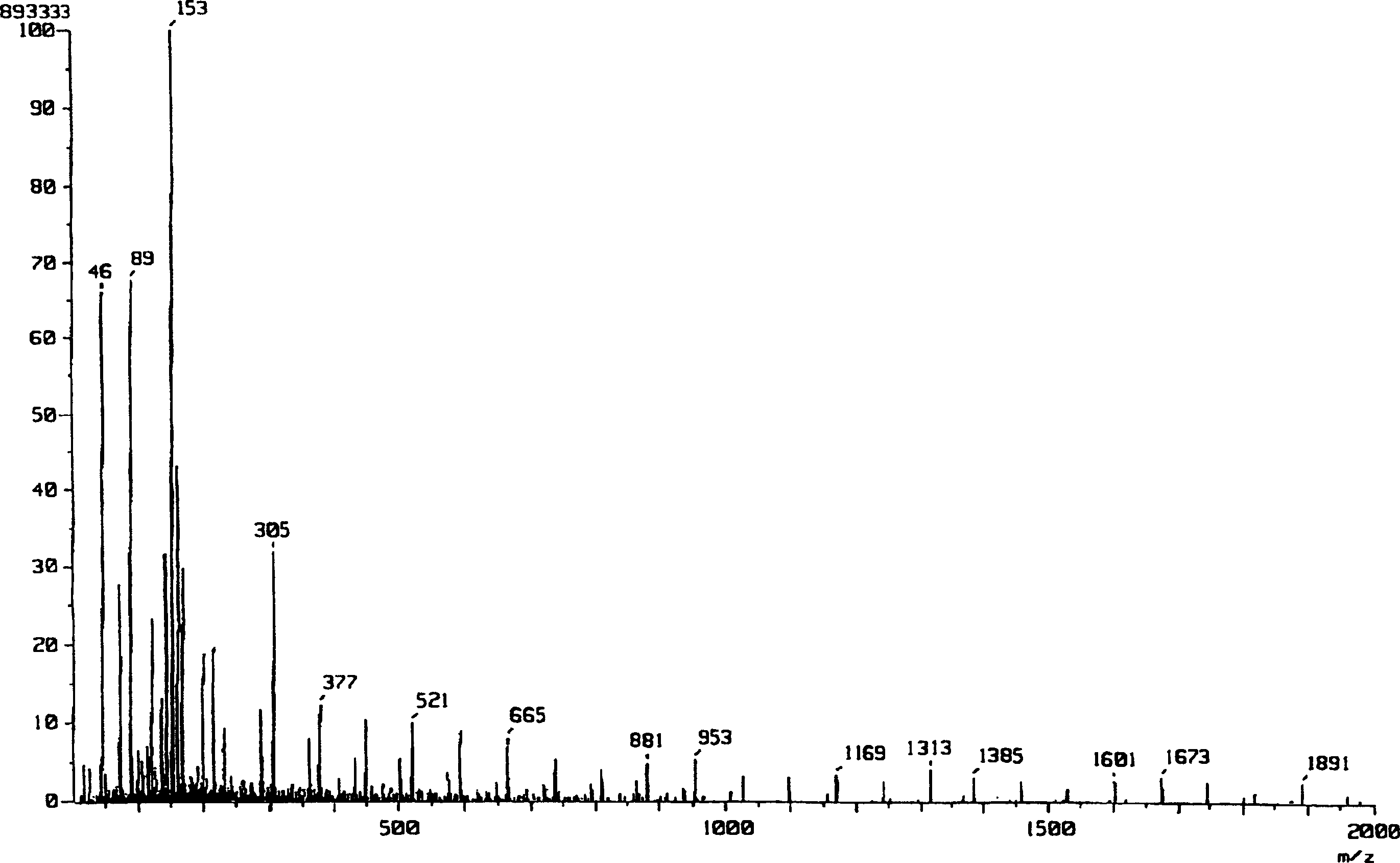
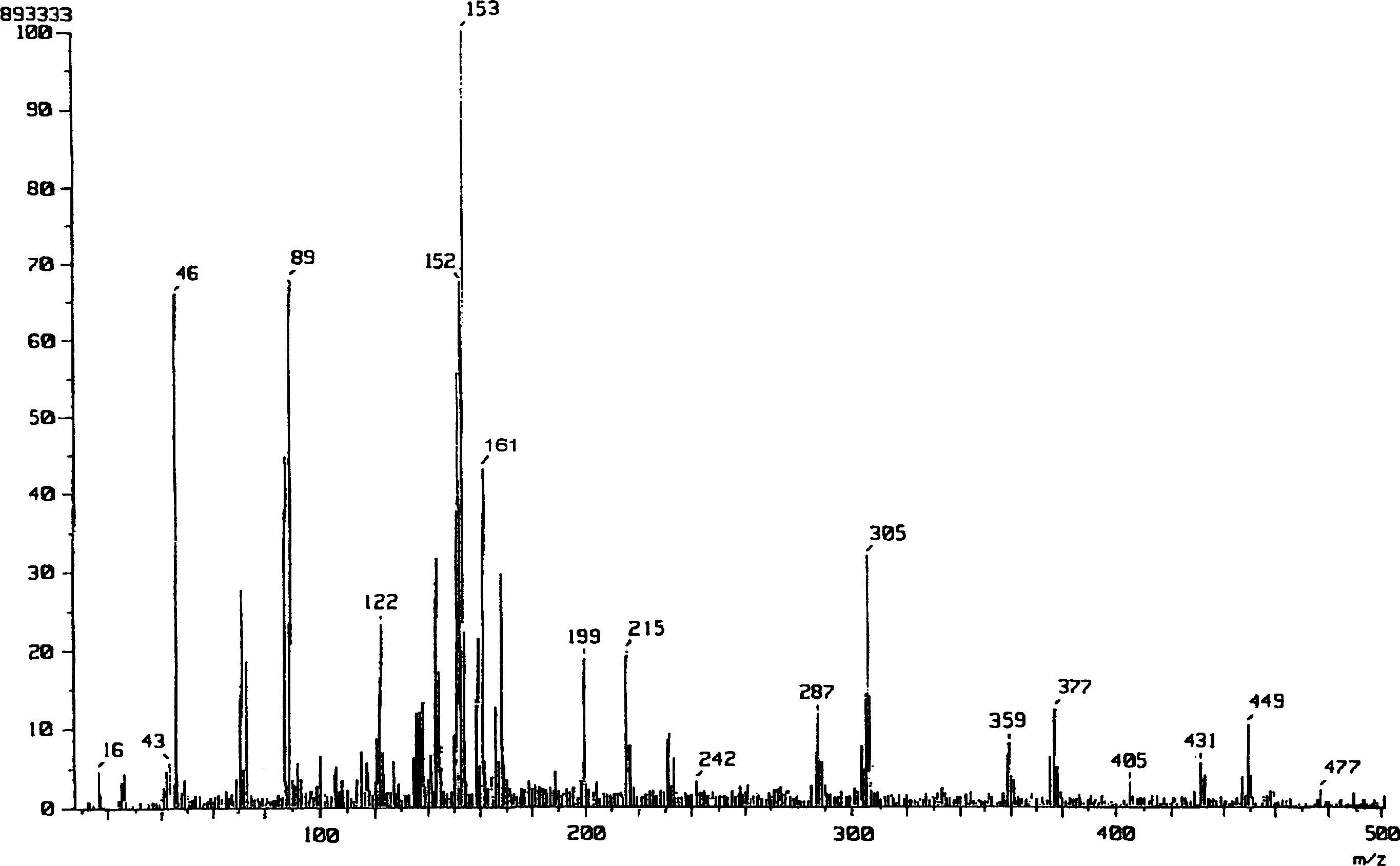
[0054] The reaction scheme of Example 1 is shown below.
[0055]
[0056] Lithium diisopropylamide (LDA)
[0057]
[0058] Add 0.63mL (1mmol) of n-butyllithium (1.6M hexane solution) to a 5mL THF solution of 0.101g (1mmol) of diisopropylamine at 0°C under a nitrogen atmosphere, and stir for 10 minutes to obtain diisopropylamine After lithium propylamide (LDA), a 4 mL THF solution of 0.577 g (4 mmol) of L-(-)-lactide was added, followed by stirring for 15 minutes to allow a reaction. To the reaction mixture was added 20 mL of saturated aqueous ammonium chloride to work up the reaction, and then 10 mL of water was added. It was extracted 5 times with THF (50 mL), and the organic layer was dried over anhydrous sodium sulfate. After filtering anhydrous sodium sulfate, the organic solvent was concentrated under reduced pressure to obtain 0.53 g of a crude product. The resulting crude product was added to 6 mL of ether, immersed in an ult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com