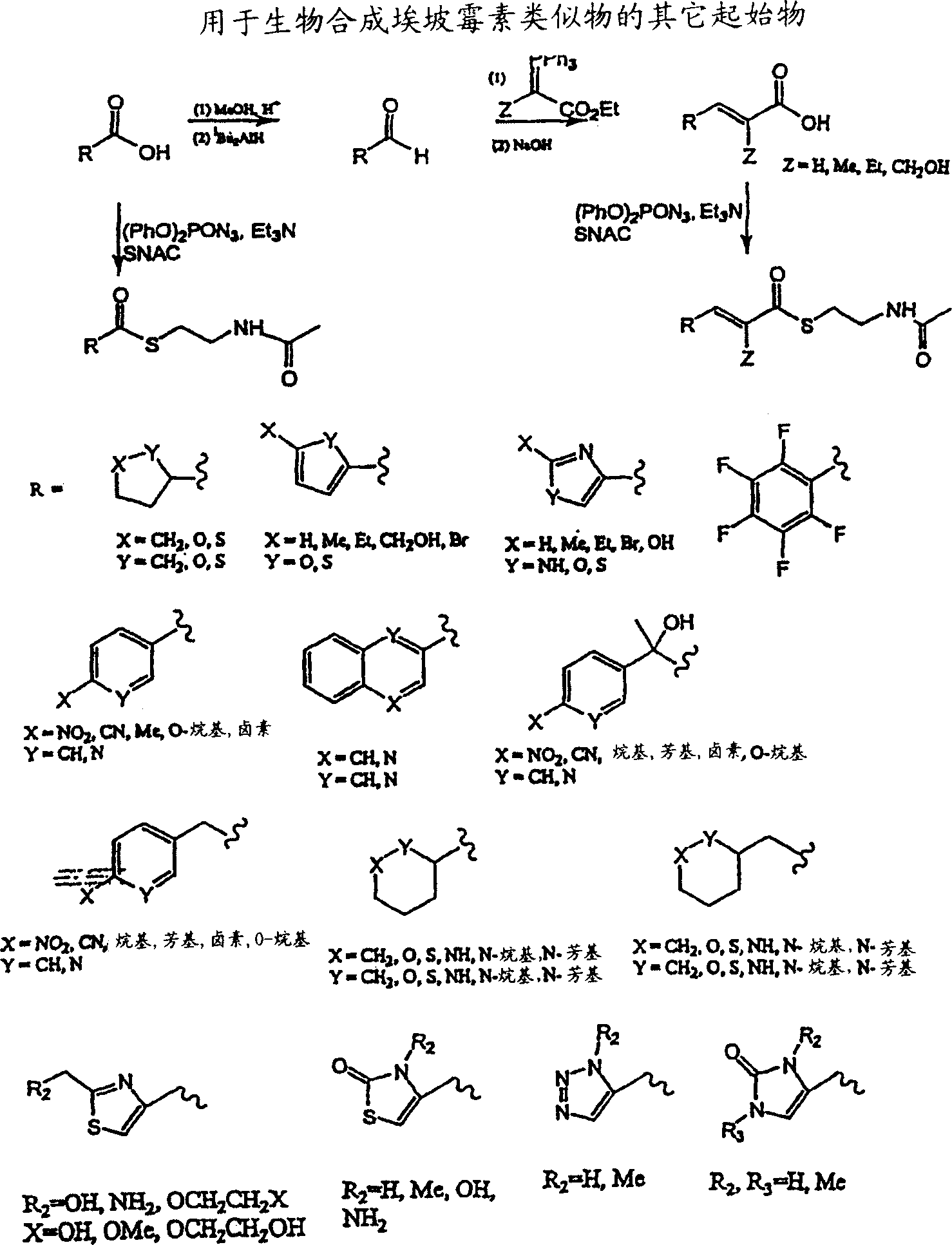
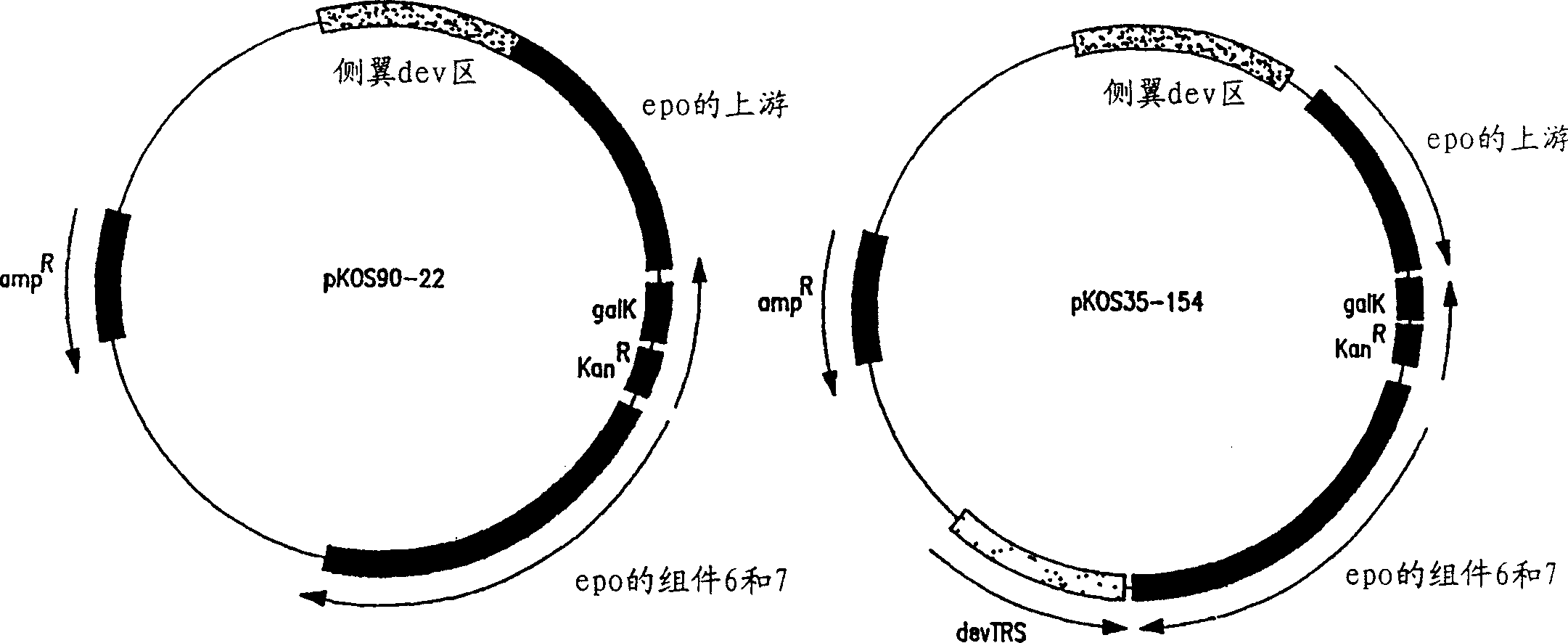
Production of polyketides
A compound, polyketone technology, applied in the fields of chemistry, medicinal chemistry, molecular biology and pharmacy, medicine, agriculture, and can solve the problems of laborious, impractical and expensive chemical synthesis of epothilone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 8
[0075]A large number of promoters are available for the preferred Myxococcus expression vectors of the invention. See Example 8 below. For example, the promoter of the S. cellulosus epothilone PKS gene (see PCT Publication No. 00 / 031247, incorporated herein by reference) functions in Myxococcus xanthus host cells. The epothilone PKS gene promoter can drive the expression of one or more epothilone PKS genes or other PKS gene products in recombinant host cells. Another preferred promoter for expressing the recombinant PKS of the present invention in Myxococcus xanthus host cells is the pilA gene promoter of Myxococcus xanthus. This promoter and two strains of Myxococcus xanthus have been described in Wu and Kaiser, December 1997, the expression regulation of pilA gene in Myxococcus xanthus, Journal of Bacteriology 179 (24): 7748-7758, and here Cited for reference, the above two strains of Myxococcus xanthus are pilA-deleted strains and pilS-deleted strains, which can express h...
Embodiment 16
[0351] Example 16 describes this synthesis in more detail.
[0352] In another embodiment, the macrolides of the invention can be converted into the macrolides of the invention. As depicted in Scheme 3, the deoxymacrolides of the present invention are epoxidized with dimethyldioxirane as previously described in Scheme 2 to provide the oxygen-containing counterparts.
[0353] Diagram 3
[0354]
[0355] The oxygenated macrolide is treated with sodium azide and tetrakis(triphenylphosphine)palladium to open the ring and form the azido acid. The azide is then reduced with trimethylphosphine to form the aminocarboxylic acid.
[0356] The epoxy compounds with NH at the W position of the present invention can be produced by macrolactamization of aminocarboxylic acids.
[0357] Diagram 4
[0358]
[0359] As shown in Scheme 4, aminocarboxylic acids are treated with 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide and 1-hydr...
Embodiment 1
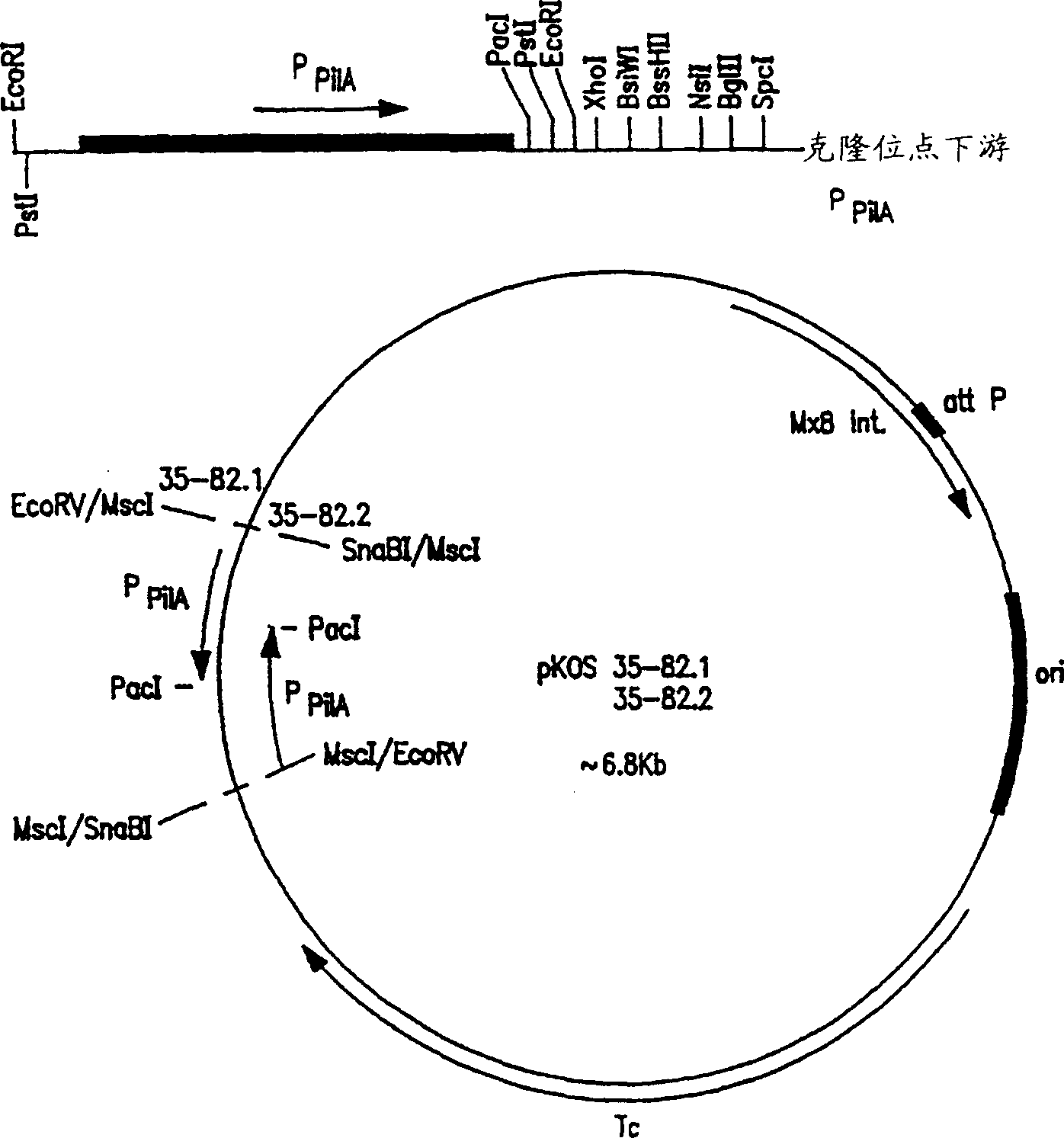
[0389] Construction of Expression Vector of Myxococcus xanthus
[0390] DNA from phage Mx8, which provides integration and attachment functions, was inserted into commercially available pACYC184 (New England Biolabs). The ~2360 bp MfeI-SmaI fragment was isolated from plasmid pPLH343 (see Salmi et al., Feb. 1998, J. Bacteriology 180(3):614-621) and ligated into the large EcoRI-XmnI restriction fragment of plasmid pACYC184. The circular DNA thus formed was ~6 kb in size and was named plasmid pKOS35-77.
[0391] Plasmid pKOS35-77 is used as a convenient vector for expressing the recombinant PKS gene of the present invention under the control of the epothilone PKS gene promoter. In an example embodiment, the complete epothilone PKS gene with its cognate promoter is inserted into a plasmid in one or more fragments to generate the expression vector of the invention.
[0392] The present invention also provides such an expression vector, wherein the recombinant PK...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com